Data Spotlight
Reliable Online Fluorescence Monitoring in CHO Cell Cultures
Background
Developing antibody-based oral vaccines requires large-scale production of monoclonal antibodies (mAbs) for in vivo studies. Outsourcing production can be costly, so many researchers turn to in-house cell culture systems to generate the necessary quantities. A key challenge in this process is the lengthy timeline—often six months or more—to identify stable, high-producing cell lines.
To address this bottleneck, researchers have leveraged a T2A peptide–based system linking antibody production with GFP expression. Because GFP and mAb are produced in equimolar amounts, intracellular GFP serves as a direct proxy for secreted antibody levels. By monitoring GFP expression, scientists can quickly select for high-producing, stable clones and track expression stability over time. When combined with temperature shift strategies in CHO-S cell culture, this approach enables higher yields and more efficient production cycles.
Results

The MPS was mounted beneath the shake flask and simultaneously monitored three key parameters in the CHO cell culture: Biomass, dissolved oxygen (DO), and fluorescence. Offline samples were taken periodically to determine cell count, cell viability, and fluorescence using a plate reader.
The first graphic shows a strong correlation between the online biomass measurements and offline cell counts during the initial 90 hours of cultivation. However, in the final ~20 hours, the online biomass signal rises more sharply than the offline data suggests. At the same time, the DO measurements indicate a slowdown in metabolic activity, as revealed by a recovery in DO partial pressure—suggesting that active cell growth has stopped. This discrepancy may be due to light scattering by accumulated cell debris, which elevates the backscatter signal and falsely suggests a steeper increase in biomass that is not supported by viable cell counts.
The fluorescence data show excellent agreement between online and offline measurements for the GFP-transfected CHO cells. As expected, the negative control (non-transfected cells) displays no significant increase in fluorescence throughout most of the cultivation period. However, a slight rise in fluorescence is detected in the negative control after approximately 125 hours. A more pronounced increase in fluorescence is also observed in the GFP-expressing culture toward the end of cultivation—exceeding what would be expected from GFP expression alone. This suggests that the late-stage fluorescence rise is not solely due to GFP, but may also be influenced by additional factors such as autofluorescence and increased scattering by smaller particles from cell debris generated during cell death. The time shift between the fluorescence increase in the negative and positive controls can likely be attributed to biological differences between the cell populations and to different cell inoculation amounts. Importantly, this late-stage fluorescence trend is also captured in the offline measurements of the GFP-transfected cells. Overall, these findings confirm that the fluorescence signal measured by the MPS is both sensitive and reliable for real-time monitoring in CHO cell cultures.
Materials & Methods
For the cultivation of CHO cells, 250 mL Corning flasks were used with a filling volume of 30%. Cultures were shaken at 140 rpm with a shaking diameter of 25 mm, and maintained at a temperature of 39 °C. Biomass was determined by monitoring the backscatter signal with the Multiparameter Sensor (MPS) on the center diode with bin 29 at 622 nm.
Fluorescence was measured at an excitation of 465 nm and an emission of 555 nm on bin 29. Offline fluorescence signals were determined in the plate reader at an excitation wavelength of 480 nm and an emission wavelength of 509 nm.
Conclusion
Using real-time monitoring of GFP expression and cell growth, researchers were able to validate that fluorescence reliably reflects antibody production, consistent with prior published findings. This proof-of-concept demonstrates how fluorescence can serve as a powerful, non-invasive marker for optimizing cell line selection and determining the optimal harvest point in antibody production workflows. By simplifying clone screening and production monitoring, the approach accelerates timelines, reduces costs, and strengthens in-house capabilities for therapeutic antibody development.
This research was conducted by:
M. Lauer and Prof. Dr. D. Lütkemeyer

Have questions about your application?
Let’s work together to find a solution that works best for you.
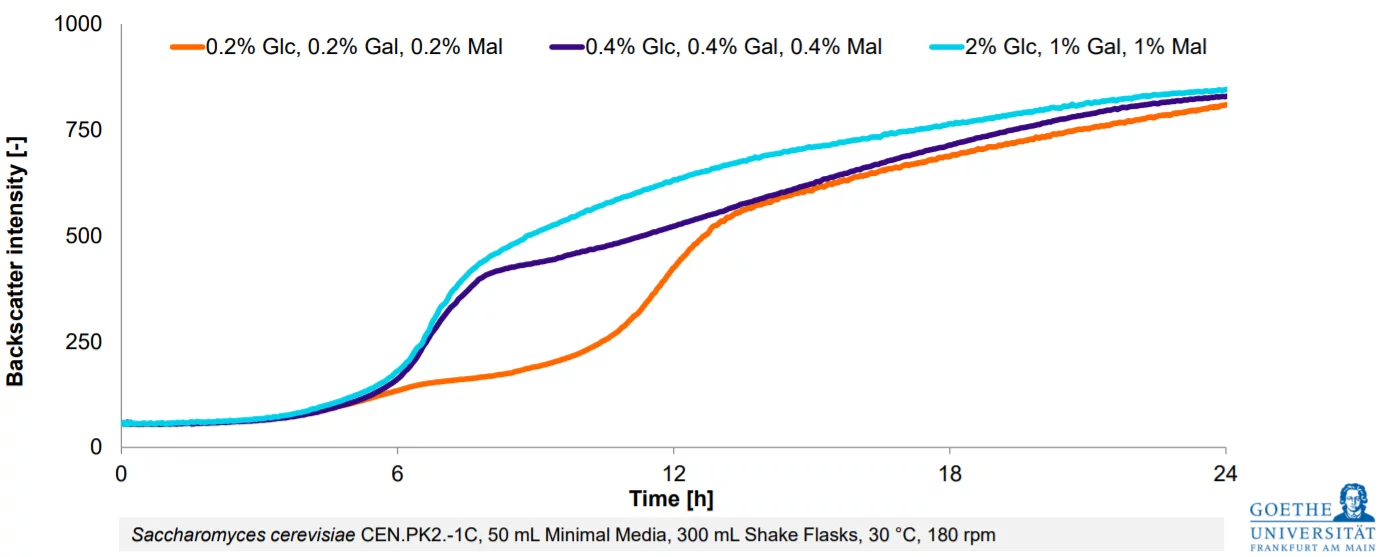
From Estimation To High-Resolution Growth Curves
Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
