.jpg)
pH Monitoring
for Suspension Cultures In Bioprocessing
Considered a Critical Process Parameter (CPP), pH is often closely monitored and controlled because it can have a dramatic impact on one's bioprocess. For maximum productivity, cells need to be cultured within a specific pH range. However, maintaining a set pH can be a challenge. Metabolic changes within microorganisms can influence the pH of their environment, as can changes in process conditioning.
What Is pH?
pH stands for the "potential of hydrogen" and measures how acidic or basic a solution is. It is a non-dimensional number and is measured on a scale of 0 to 14, with 7 being neutral. Values lower than 7 are considered acidic; values greater than 7 are basic or alkaline.
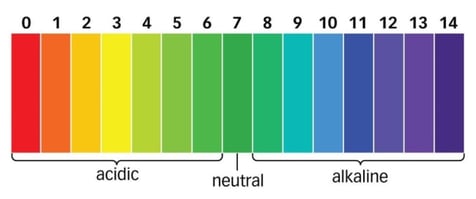
pH is the the inverse decadic logarithm of the concentration of hydrogen ions in an aqueous solution. The more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH.
pH Ranges in Bioprocessing
Not only can pH influence cells directly through changes in cellular morphology, microbial activity, and biological functions, but it can also impact factors outside of the cells such as the availability of nutrients and the properties of chemicals.
Most animal cells, mammalian included, are kept between a pH value of 7.2 to 7.4. However, these ideal values can vary depending on the cell type. For example, transformed cells, those that have been genetically altered, tend to prefer slightly lower pH values of 7.0 while unaltered fibroblast cell lines do better at slightly alkaline pH values between 7.4 and 7.7. Insect cells, on the other hand, have an optimum pH of 6.2.
Microbes can grow in different environments, in part, by adapting to the pH of their habitat. Most bacteria grow over a a pH range of 3 units. When it comes to the relation towards pH, microorganisms can be divided into three main groups:
- Acidophiles are organisms are those that thrive under highly acidic conditions, less than pH 5.5. Some examples are Leptospirillum ferriphilum and Sulfolobus acidocaldarius.
- Neutrophiles live and thrive in an environment with a relatively neutral pH ( pH 5.5 to 9). Some of the most common include Escherichia coli, Staphylococcus aureus, and Lactobacillus acidophilus.
- Alkaliphiles are microorganisms that grow well at pH values exceeding pH 9. An example of this group is Halorhodospira halophila.
Why Monitor pH?
Changes in Cellular Morphology and Function
All cells have an optimal pH level that supports their growth. While this can vary some, most cells prefer to be around neutral. When pH levels fall outside of the acceptable range, cell culture growth can become inhibited or altered and the titer of their products reduced. pH levels that are too acidic or alkaline can actually destabilize the genetic material of cells, which can cause mutations and ultimately cell death if not corrected.
Indication of Contamination
It is not possible to visualize contamination in clear media, however, sudden changes in pH are a good indicator of bacterial or fungal contamination. Most cases of bacterial contamination in the cell culture laboratory are caused by aerobes.
When working with indicator dyes, the media will become acidic and appear yellow if contaminated with aerobic bacteria. However, if the bacteria are anaerobic, the contamination will cause the medium to become basic and will appear pink. A fungal contamination will cause the media to turn hazy.
Contamination is a concern because bacteria often produce toxins that disrupt cell function and can ultimately destroy cell cultures. Cell cultures are especially susceptible to contaminations because they grow much slower than most bacteria and fungi, so the latter easily overgrow mammalian cells in nutrient rich media.
.png?width=2352&height=2429&name=pH-Flasks-Yellow-and-Pink-Medium%20(1).png)
Availability of Nutrients
Culture media, also known as growth media, is any solution comprised of amino acids, vitamins, inorganic salts, glucose, and other substrates that support the health and growth of cells in culture. Many also contain serums as a source of growth factors, hormones, and attachment factors.
Depending on the cell type and preferred outcome, nutrient supplementation in cell culture can be used to increase cell viability and genomic stability. The surrounding pH can influence how cells take in these nutrients. Macronutrients such as nitrogen, potassium, calcium, magnesium, and sulfur are more readily available at pH 6.0–6.5, while micronutrients become less available at higher, alkaline levels of greater that 7.0.
Why Does pH Change?
While critical to the success of every bioprocess, pH proves challenging because it is closely intertwined with cell culture conditions and is affected by changes at both, the cellular level and within the bulk environment.
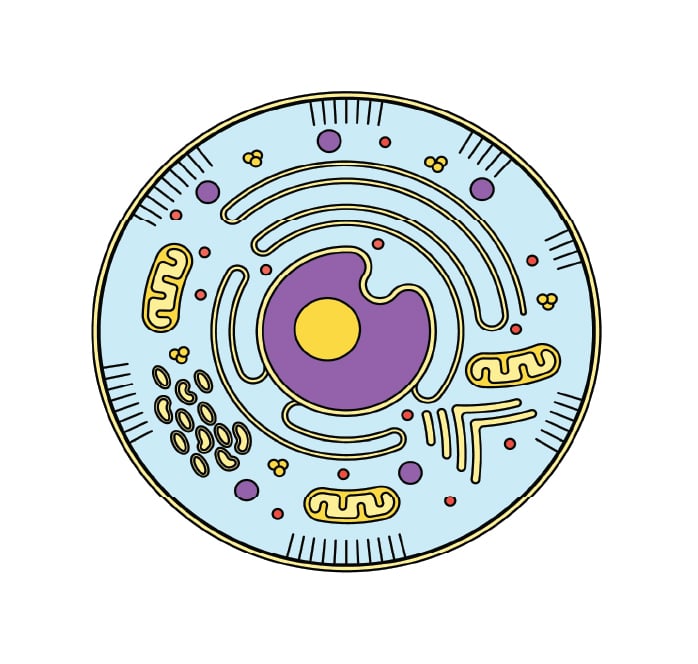
Cellular Processes
In bioprocessing, living organisms are used to convert one or several substrate/s into a desired product. These products can influence the pH of your culture environment.
The problem of drifting pH is often very pronounced in microbial cultures, especially in aerobic processes with oxygen limitation, where under low oxygen the cells start to use alternative metabolic pathways, such as mixed acid fermentation, which heavily acidifies the media and leads to strong growth inhibition.
Also microbial growth on complex media can be challenging regarding pH, e.g. if the main carbon source is protein and amino acids, the pH will drift to the basic region due to the accumulation on ammonia.
All that is one of the reasons, why fed batch cultures usually outperform batch-cultures, as you can avoid these pH drifts by feeding the same amount of nutrients in a properly controlled way and thereby preventing accumulation of overflow metabolites.
As cells grow and cell density increases, the problem of acidification increases along with the production of these acidifying molecules.
Culture Environment
The survival of your culture is closely intertwined with environmental factors, e.g. temperature, pressure influencing the solubility and dissociation of pH-active gases (CO2, ammonia).
Not only does temperature independently play an important role in cell growth as cells have a preferred temperature range, it can also influence the pH levels. For example, under standard conditions (25°C and 1 atm), the pH of pure water is 7. At 30°C, the pH drops to 6.92. This is because at higher temperature, water dissociates into ions more, leading to a higher concentration of hydrogen ions (or H3O+). A higher concentration of H3O+ ([H3O+]) results in lower pH. According to the Bronsted-Lowry acid-base theory, the sample is still in equilibrium and has not changed acidity. We must think about the temperature we are keeping our culture at but also possible temperature changes during handling.
Another consideration is shifts in gas concentration during sample handling. Atmospheric CO2 can alter the system during handling. Usually the atmospheric CO2 is lower than in the incubator. During handling, CO2 leaves the media when exposed to the external air, thereby reducing the buffering capacity and making the pH drift.
How Is pH Controlled?
Operating your bioprocess at a metabolic optimum is the best way to control the pH, i.e. by prevention of oxygen limitations, proper atmosphere selection, proper mixing, proper media selection, proper feeding strategies. An optimal bioprocess does not need pH control.
Most media contain buffering components which are designed to help stabilize pH. However, these components alone are not always sufficient to control pH. Other methods, alone or combined, can be used throughout production to help maintain desired pH levels. Or influence cell growth negatively when used in higher concentrations. Another drawback of buffers is there price, which is actually relevant for white biotech processes, which create cheap bulk products and cannot use expensive buffers.

Buffering Media
In this traditional method of pH control, a buffer is added to the media to maintain a stable value. A pH buffer acts as either a weak acid or a weak base to ensure that the media will be resistant to changes in pH. This works because the buffer is capable of donating or accepting hydrogen ions which are responsible for establishing pH. The most common base used in mammalian cell culture is sodium bicarbonate (NaHCO3).

Adding Acid/Base
When pH drifts outside the optimum range, an acid or base can be added to increase or decrease the pH as needed to bring it back to the target value. In a closed-loop setup, the amount added via the integrated injection systems (pumps, LIS, CO2) is based on feedback from automatic pH sensors. Solution is added to the process and the pH value is closely monitored.
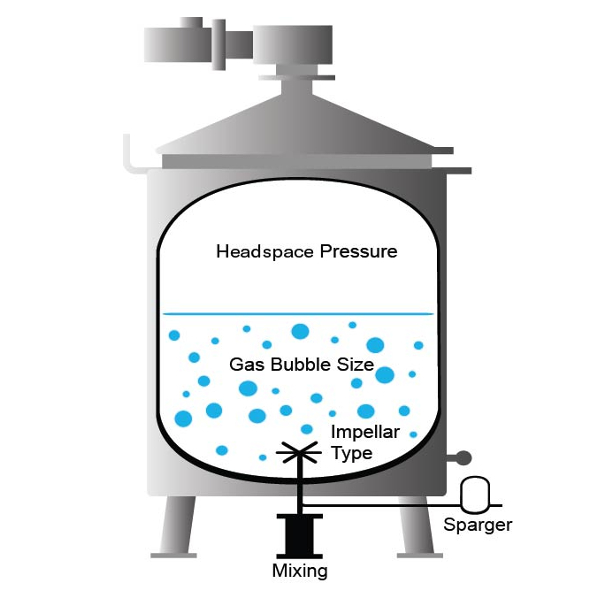
Sparging Gases
Gases such as nitrogen and oxygen can be slowly added from the bottom of a bioreactor. The gas bubbles remove dissolved carbon dioxide from the media while releasing oxygen into the solution. However, the efficiency of this method can depend on the bioreactor itself. While sparging may work with smaller bioreactors, larger units may require higher levels of agitation to help distribute the gas bubbles. This higher agitation can have an adverse effect due to increased shear on the suspended cells.
How Is pH Measured?
- pH Indicator Dye
- pH Probe
- Fiber Optic Sensor

Principle of Measurement: Colorimetric
Most commercially available cell culture media can be purchased with added pH indicator dyes. The most common dye, phenol red (PR, also known as phenolsulfonphthalein) gradually transitions from yellow to red over a pH range of 6.2 to 8.2. The pH level is determined by matching the color of the solution to a color chart.Technologies:
Inert addition to culture media, compatible with all vessel types-
Advantages
- Widely accepted in cell biology
- Easy to use - requires little technical knowledge
- Inexpensive - any costs are usually associated with other consumables
- No additional equipment needed
- Compatible with all vessel types
-
Disadvantages
- Does not provide an exact measurement
- Not accurate due to color subjectivity
- Can be influenced by particles
- Chemical formula of indicator dyes can mimic certain hormones
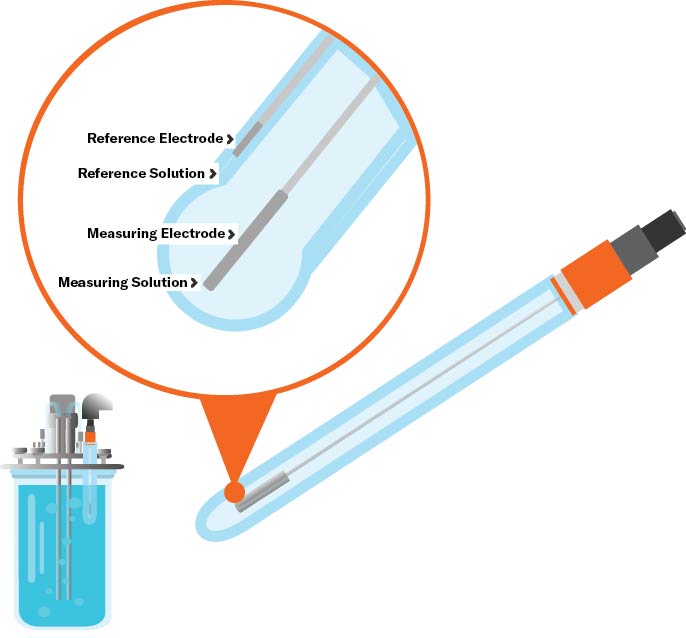
Principle of Measurement: Potentiometric
A typical pH electrode consists of a sensing element and a reference element (which contains a buffer solution of pH 7). A thin glass membrane, or junction, separates the two elements and is permeable by hydrogen ions. When placed in solution, the hydrogen ions migrate to maintain equilibrium between the sensing and reference elements. As the ions move in or out of the pH electrode, an electrical potential is created, which is measured by a pH meter that converts the voltage to a pH value.Technologies:
Used in invasive probes to spot check small vessels, or for continuous measurements in bioreactors.-
Advantages
- Automated (measurement and data handling)
- Good measurement range without the need for dilutions
- Options for high resolution data
- Applicability to a broad variety of vessel types (μL to L scale)
-
Disadvantages
- Requires regular maintenance to ensure accurate readings
- Short measuring intervals to monitor contentiously over the entire process
- Can have a long response time
- Medium initial investment (CAPEX) for meter and probe
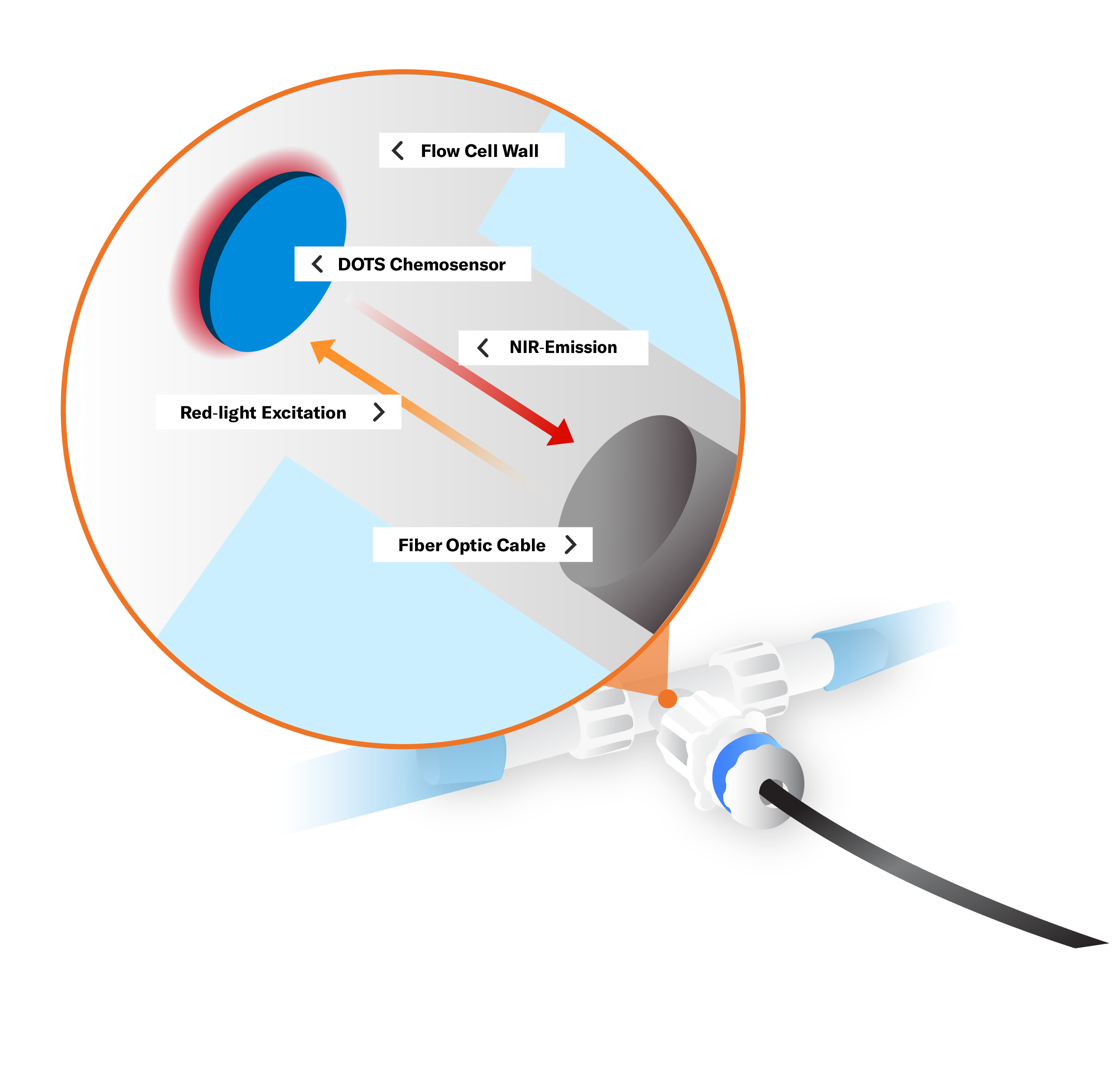
Principle of Measurement: Spectroscopy - Luminescence
A chemosensor containing pH luminescent dye indicators is embedded in a matrix. A sensor emits light at one wavelength, exciting the chemosensors which show luminescence in another wavelength. Depending on the concentration of hydrogen ions present in the solution, the amount of luminescence changes. The sensor measures this phase shift which is then calculated to represent a pH value.Technologies:
Integrated into bioreactor systems via on-line flow loops (including harvest lines, sampling lines, media in/out flow lines, waste removal lines)-
Advantages
- Online and non-invasive (no samples need to be taken)
- Automated (measurement and data handling)
- Continuous real-time data
- Little to no maintenance
- Works with a variety of vessel types
- High resolution data
-
Disadvantages
- Sufficient flow is needed for accurate measurements
- Running costs for consumables (OPEX)
- Not compatible with all setups

Principle of Measurement: Colorimetric
Most commercially available cell culture media can be purchased with added pH indicator dyes. The most common dye, phenol red (PR, also known as phenolsulfonphthalein) gradually transitions from yellow to red over a pH range of 6.2 to 8.2. The pH level is determined by matching the color of the solution to a color chart.Technologies:
Inert addition to culture media, compatible with all vessel types-
Advantages
- Widely accepted in cell biology
- Easy to use - requires little technical knowledge
- Inexpensive - any costs are usually associated with other consumables
- No additional equipment needed
- Compatible with all vessel types
-
Disadvantages
- Does not provide an exact measurement
- Not accurate due to color subjectivity
- Can be influenced by particles
- Chemical formula of indicator dyes can mimic certain hormones

Principle of Measurement: Potentiometric
A typical pH electrode consists of a sensing element and a reference element (which contains a buffer solution of pH 7). A thin glass membrane, or junction, separates the two elements and is permeable by hydrogen ions. When placed in solution, the hydrogen ions migrate to maintain equilibrium between the sensing and reference elements. As the ions move in or out of the pH electrode, an electrical potential is created, which is measured by a pH meter that converts the voltage to a pH value.Technologies:
Used in invasive probes to spot check small vessels, or for continuous measurements in bioreactors.-
Advantages
- Automated (measurement and data handling)
- Good measurement range without the need for dilutions
- Options for high resolution data
- Applicability to a broad variety of vessel types (μL to L scale)
-
Disadvantages
- Requires regular maintenance to ensure accurate readings
- Short measuring intervals to monitor contentiously over the entire process
- Can have a long response time
- Medium initial investment (CAPEX) for meter and probe

Principle of Measurement: Spectroscopy - Luminescence
A chemosensor containing pH luminescent dye indicators is embedded in a matrix. A sensor emits light at one wavelength, exciting the chemosensors which show luminescence in another wavelength. Depending on the concentration of hydrogen ions present in the solution, the amount of luminescence changes. The sensor measures this phase shift which is then calculated to represent a pH value.Technologies:
Integrated into bioreactor systems via on-line flow loops (including harvest lines, sampling lines, media in/out flow lines, waste removal lines)-
Advantages
- Online and non-invasive (no samples need to be taken)
- Automated (measurement and data handling)
- Continuous real-time data
- Little to no maintenance
- Works with a variety of vessel types
- High resolution data
-
Disadvantages
- Sufficient flow is needed for accurate measurements
- Running costs for consumables (OPEX)
- Not compatible with all setups
sbi's pH Monitoring Solutions
Scientific Bioprocessing (sbi) is dedicated to pioneering Digitally Simplified Bioprocessing. We develop cutting-edge digital technologies (e.g., sensors, actuators and software) that simplify bioprocessing activities from the lab to the production floor.
Our pH monitoring solution features fiber optic sensors and software that:
-
Detect changes in environmental conditions early and ensure a healthy cell culture environment.
-
Save you hours of manual, hands-on time required for pH probe setup, calibration, and conditioning.
-
Simply connect the flow cells directly into your flow loops (e.g., feeding, sampling or harvesting flow lines for bioreactors) and start measuring.
Our pH Monitoring Solutions

pH Flow Cells For Flow Loops
-
Continuously monitor pH in flow loops using fiber optic sensors.
-
pH for a variety of applications: 5-7, 6-8, 7-9
-
Flexible flow rate range: 5-500 mL/min
-
Factory-calibrated and pre-sterilized
-
Single-use design reduces risk of contamination
Compatible with:
-
Perfusion bioreactors, custom benchtop bioreactors, and small-scale fermenters
-
On-line flow loops: Harvest lines, sampling lines, media in/out flow lines, waste removal lines
View The Success Stories
-- Kitana Manivone Kaiphanliam (Washington State University)

