1. CELL GROWTH QUANTIFIER (CGQ)
Measure biomass in your shake flask with the CGQ in real-time and visualize your data in the DOTS Software.

Biomass-based feeding describes the feeding of a substance to a growing culture initiated by the reach of a certain biomass level. It offers a new and advanced control option in comparison to other modern feeding techniques. This brand-new application for shake flasks is enabled by the interconnectivity of a reliable biomass monitoring sensor, a state-of-the-art automated feeding system, and a powerful software.
While automated feeding has been commonly realized in bioreactors for many years already, this mechanism is used more frequently in shake flasks only recently, thanks to advancing techniques. A controlled feeding on a shake flask level only became possible lately, by sbi's Liquid Injection System (LIS) technology. Having certain automated feeding options in shake flasks enhances the control options of small-scale cultivations and offers a new range of applications for this vessel type.
Time-based automated feeding, starts the injection of liquid at defined time-points, avoiding the necessity of manual labor outside normal office hours, improving work conditions for many scientists. The introduced liquids can be diverse – from substrate feeding, enabling fed-batch or continuous cultivations in shake flasks to the injection of chemicals to test their effect on the cell’s metabolism or to modify culture conditions, like the pH or foaming of the medium.
The options of time-based automated feeding vary but usually require good knowledge of the culture to define the best feeding time-points.
Biomass-based feeding, cell growth triggered liquid injection, or substance release initiated by cell density values - however you might call it - allows an initiation of liquid injection that is dependent on cell growth and therefore more fine-tuned to the needs of the cultivation.
Biology is diverse by definition and even if tried intensively, no two cultures are the same. Letting the cells define the time-point of feeding is an elegant attempt to harmonize cultures.
Bioreactors, shake flasks, microtiter plates - automated feeding is a desired technology for all kinds of vessels for diverse applications. A range of techniques offer feeding options in most vessel types, today.
For large-scale productions, benchtop bioreactors or stand-alone bioreactors of volumes up to hundred thousands of liters are used, and often fed-batch conditions are applied to achieve high production yields. Most bioreactor systems possess several ports to introduce sensors for process control and tubing to feed substrate or other substances.
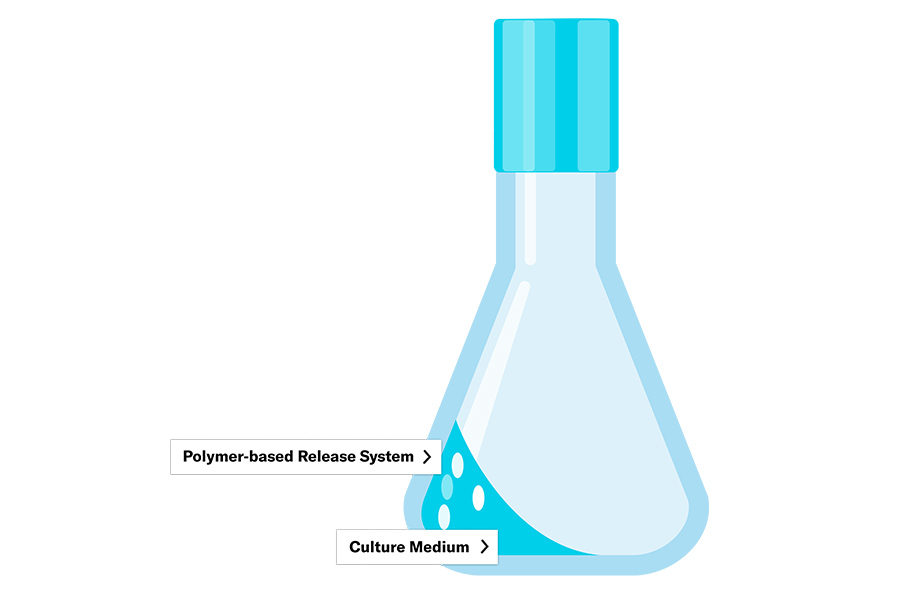
High-throughput screenings require a flexible scalability to test multiple conditions. Microtiter plates provide multiple separate reaction rooms with small volumes to test simultaneously. Feeding options in microtiter plates exist as microfluidic plates, with tiny liquid reservoirs that can be pumped into the wells. Or with integrated polymers that continuously release substrate (see polymeric delivery systems).

An enzymatic glucose release system has been developed in which glucose is enzymatically released from starch, embedded in a polymer. The process is started manually by adding the enzyme (glucoamylase) at a desired time point. This system enables growth control of processes in smaller scales (e.g., shake flasks) with glucose as a substrate.

For large-scale productions, benchtop bioreactors or stand-alone bioreactors of volumes up to hundred thousands of liters are used, and often fed-batch conditions are applied to achieve high production yields. Most bioreactor systems possess several ports to introduce sensors for process control and tubing to feed substrate or other substances.


High-throughput screenings require a flexible scalability to test multiple conditions. Microtiter plates provide multiple separate reaction rooms with small volumes to test simultaneously. Feeding options in microtiter plates exist as microfluidic plates, with tiny liquid reservoirs that can be pumped into the wells. Or with integrated polymers that continuously release substrate (see polymeric delivery systems).

An enzymatic glucose release system has been developed in which glucose is enzymatically released from starch, embedded in a polymer. The process is started manually by adding the enzyme (glucoamylase) at a desired time point. This system enables growth control of processes in smaller scales (e.g., shake flasks) with glucose as a substrate.

sbi's Liquid Injection System (LIS) is the first technology of its kind allowing for controlled, automated feeding in shake flasks. The LIS Drive connects to a sterile cartridge, a reservoir for up to 25 mL of liquid. The coupled Drive and cartridge attach to the shake flask and the Drive pumps air through a sterile filter into the cartridge, causing liquid to be dispensed into the flask. LIS offers different feeding profiles from constant to exponential feeding, enabling diverse experiments, like fed-batch cultures, pH control, or automated induction.
Every cultivation is different and one feeding profile can never fit all solutions. Depending on the process and the desired outcome, different profiles are required. The Liquid Injection System (LIS) offers diverse feeding profiles to address the special requirements of your cultivation. With the "delay" option, it is always possible to introduce feeding breaks at any time point during your experiment, enabling great flexibility in designing the feeding profile.
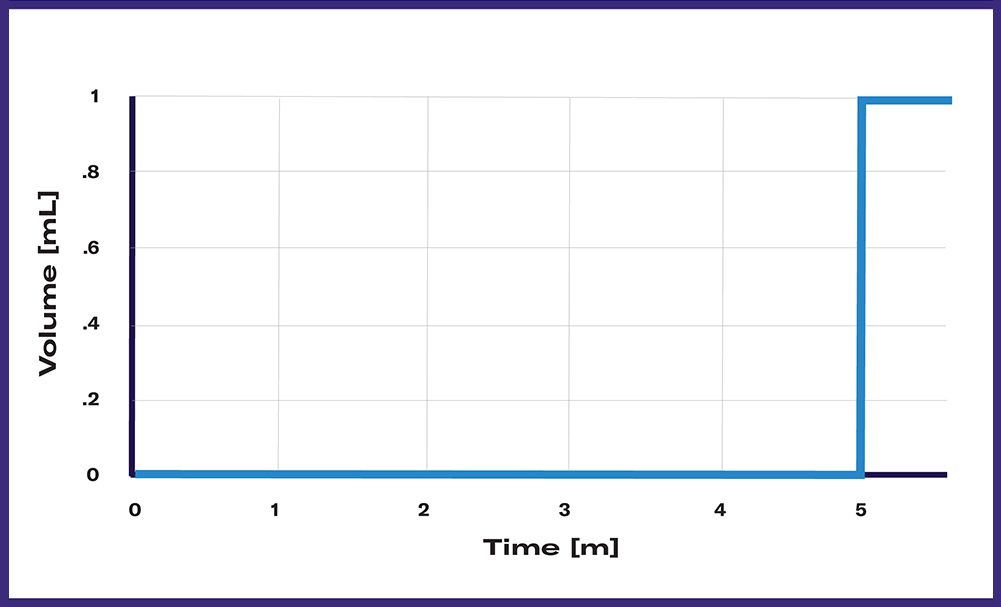
The Single Shot profile represents one single feeding event during the cultivation. The feeding event (volume and time) is programmed wirelessly with the DOTS Software and will start automatically at the desired time point. It is the ideal program for e.g., promotor induction, cell inoculation, or many more one-time injection applications.
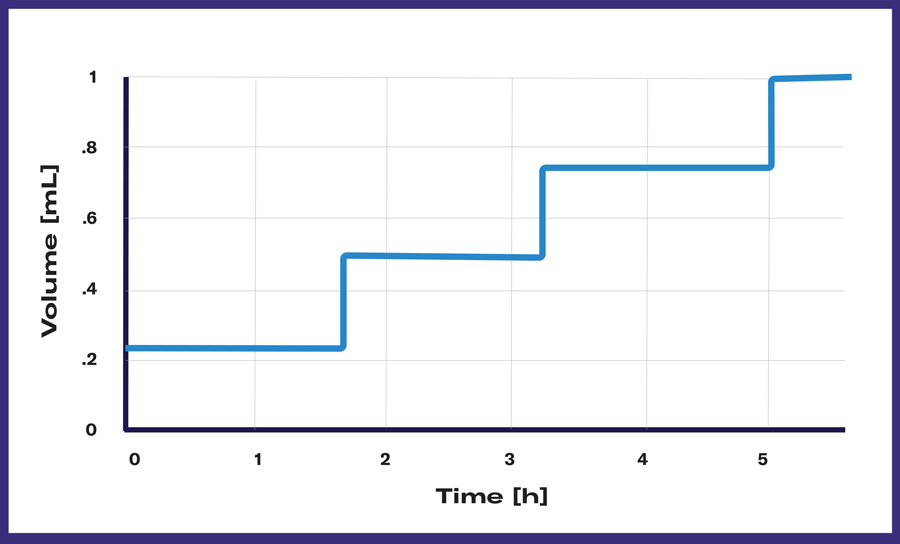
Multi Shots are useful for recurring feeding events. Preset the starting volume of liquid in the cartridge and the desired number of shots to define feeding intervals. Let your cultivation run without manual interventions and let LIS feed the programmed volume at the preset time slots, reliably. Fed-batch cultivations or toxicity tests can easily be performed with this profile, among other applications.
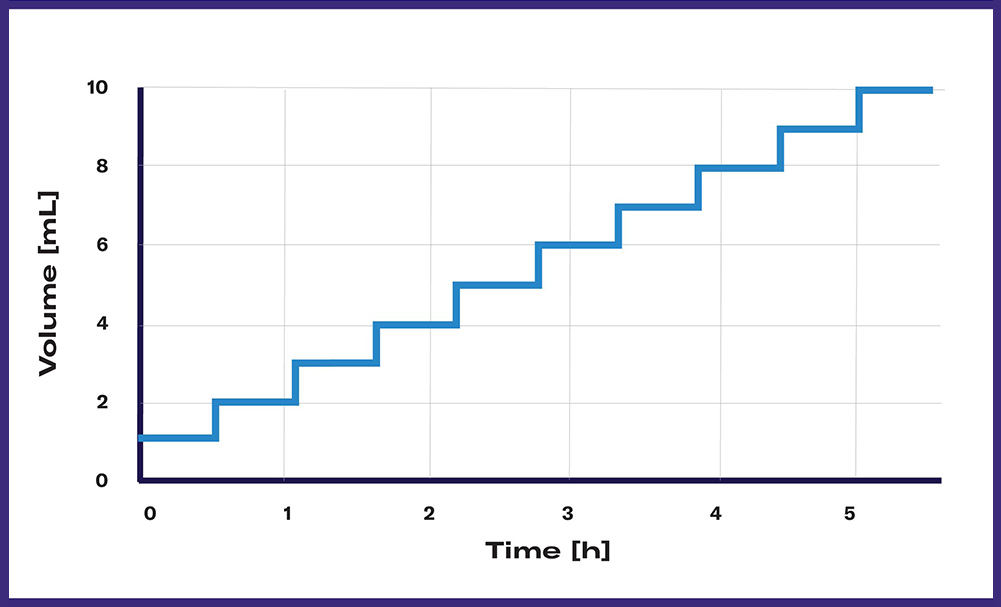
Constant feeding will inject a certain volume equally over the duration of the cultivation. Determine the total volume of liquid in the DOTS Software and the anticipated duration of your experiment and monitor the regular feeding process. With a constant substrate release (maximal: 1 mL/min), growth control of cultures can be achieved which is beneficial for many applications.
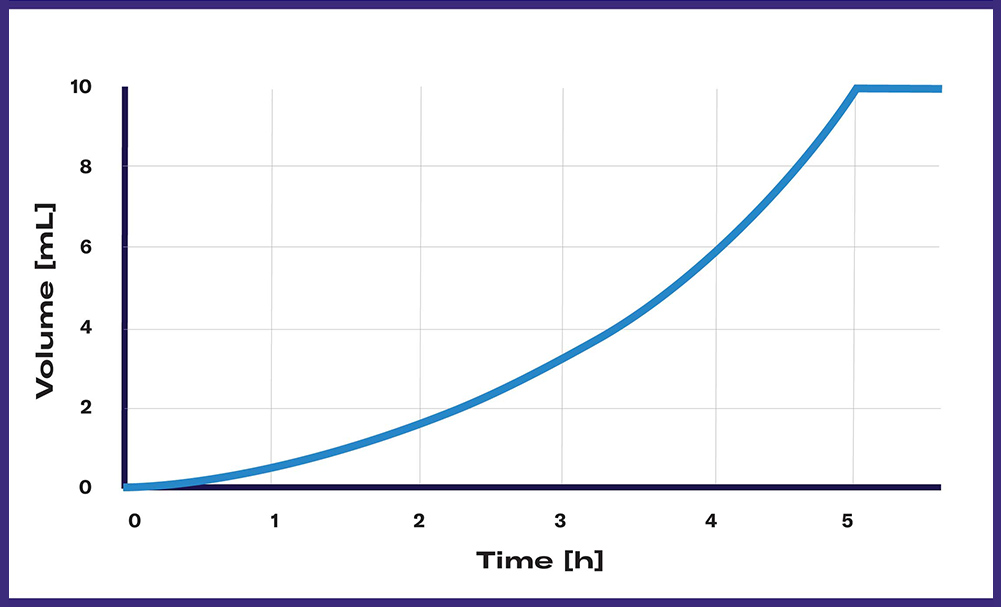
Exponential feeding describes the exponential increase of the amount of liquid released over time. Starting with minor quantities (minimum: 100 µL droplet) and steadily increasing until the largest volume is released to the flask. With fast growing cultures, a steady increase in substrate feed can support fast biomass production.
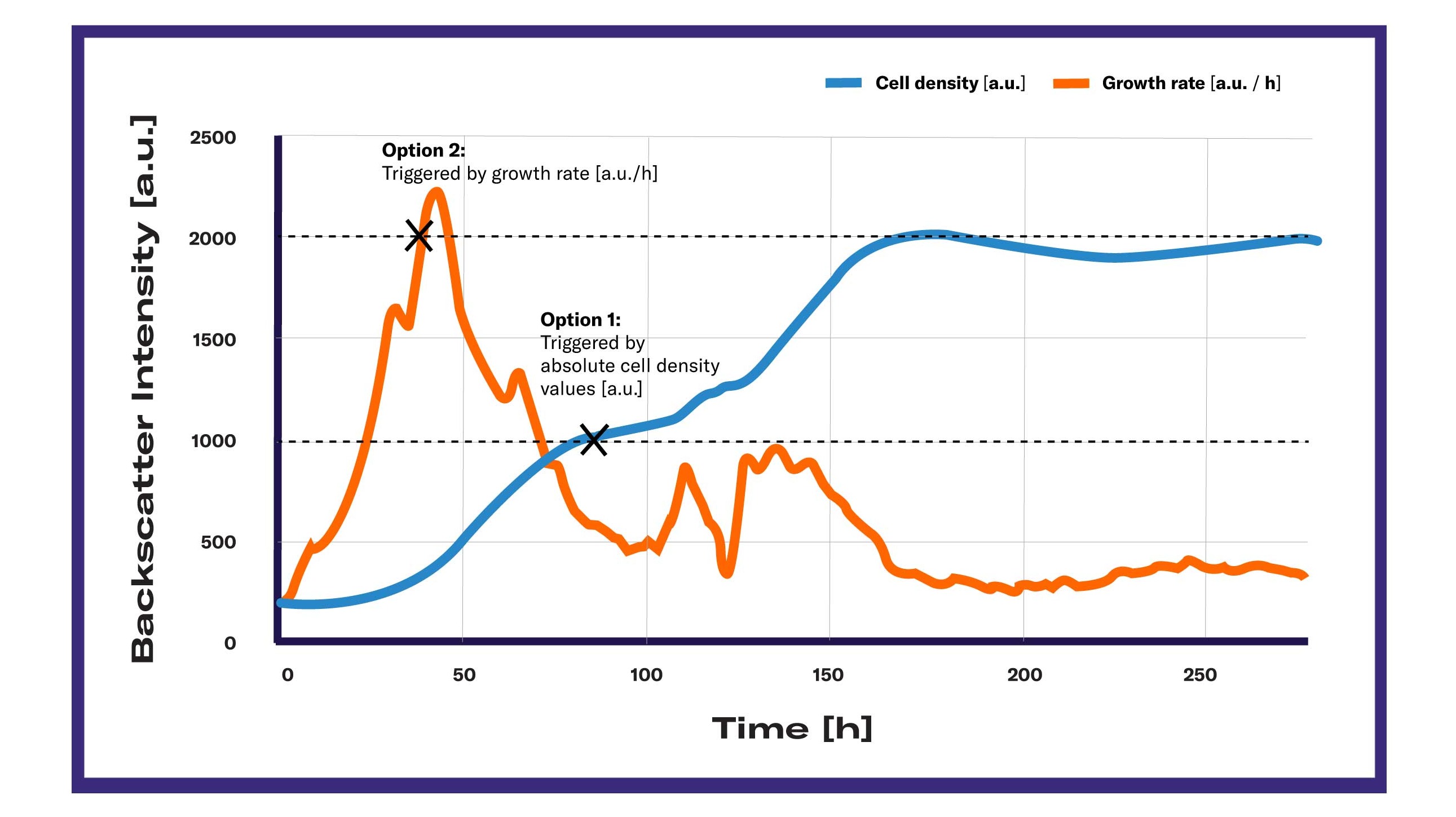
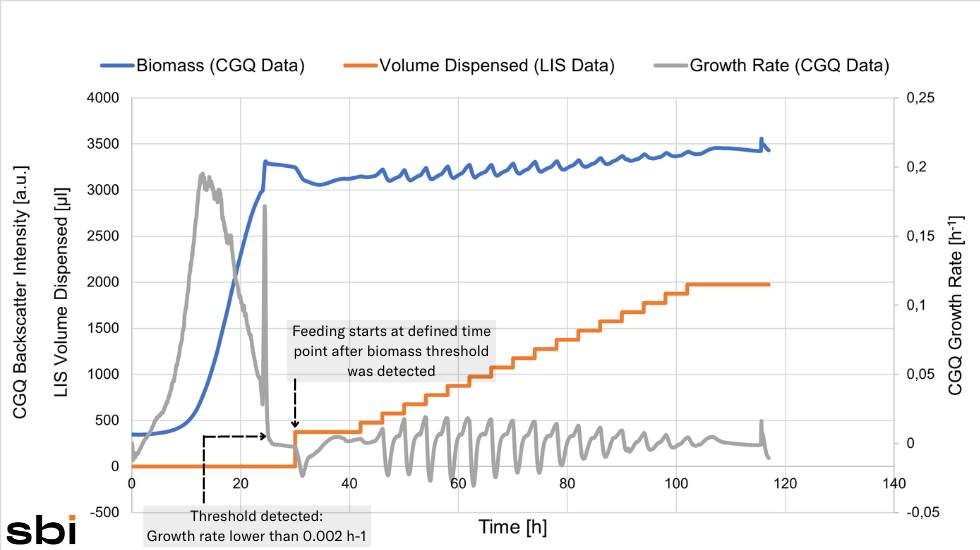
A cell growth triggered liquid injection in shake flasks offers a range of new applications and improves the control options in this vessel type. Large scale bioprocesses in bioreactors usually offer the option of automated fed-batch processes which basically rely on a cell density coupled automated feeding of substrate. In these large vessels, substrate release is mostly controlled by the oxygen partial pressure (pO2), a parameter that indirectly points out the growth rate status.
The term biomass-based feeding describes the automated substance release initiated by actual cell density values or growth rates and is therefore a novelty across vessel types.
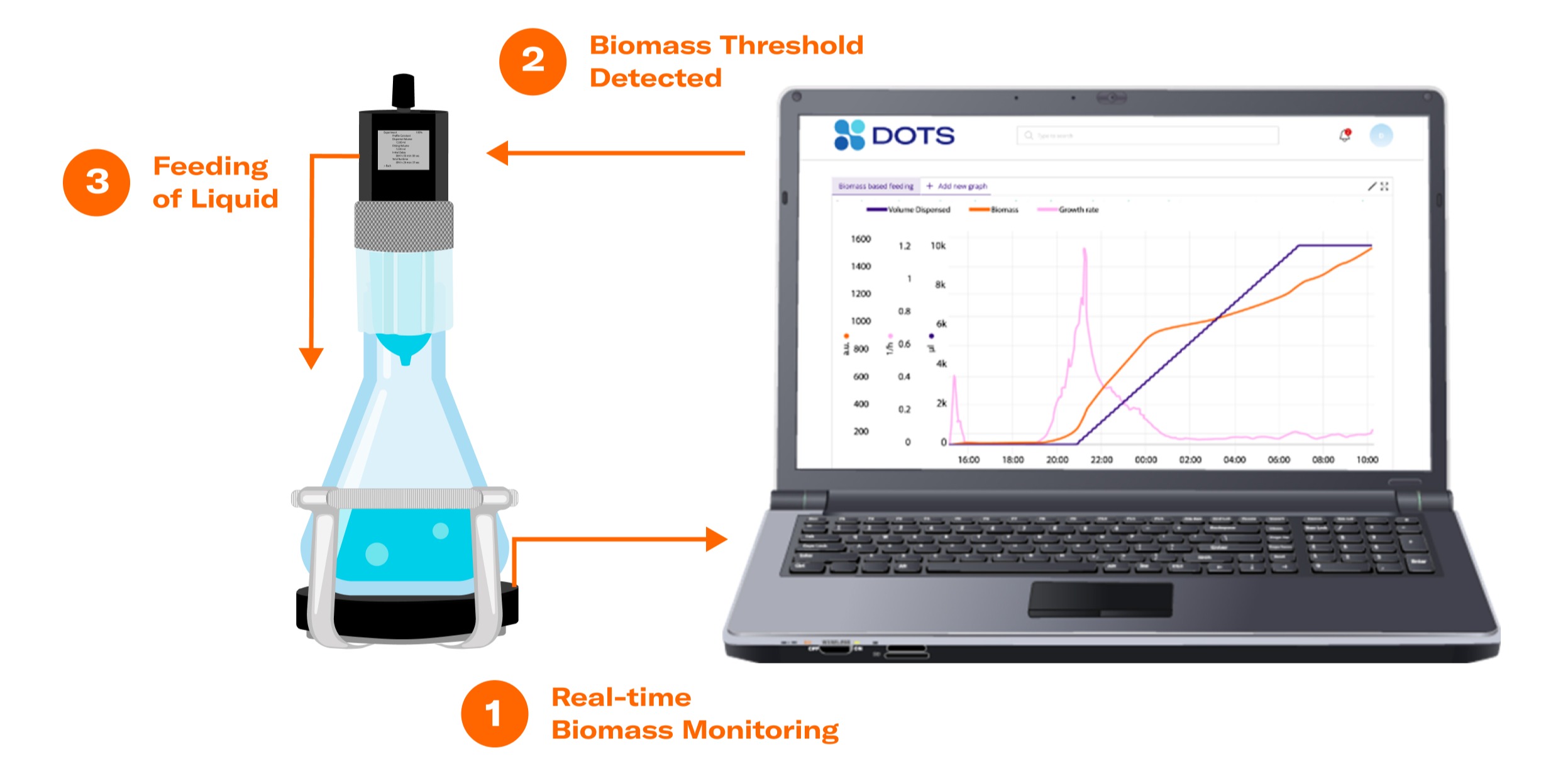
Measure biomass in your shake flask with the CGQ in real-time and visualize your data in the DOTS Software.
Preset your biomass threshold in the DOTS Software. When it is detected, the software will initiate the feeding.
LIS receives the information from DOTS Software to start feeding and injects liquid into the shake flasks according to the preset feeding profile.
"Using biomass-based feeding in shake flasks allowed me to start the methanol induction, once the cell growth phase of my Pichia pastoris culture was successfully completed and a sufficient biomass level was reached."
Dr. Christina Dickmeis, Senior Research Scientist

Measure biomass online and non-invasively through the glass wall of the flask
Monitor many flasks in parallel
Compatible with:
A broad range of microorganisms (such as bacteria, yeast, anaerobics, thermophile, or filamentous organisms)
Most shake flask sizes (100 mL – 5000 mL)
All incubation shakers (clamps and sticky mats)

Fed-batch processes are often advantageous in comparison to batch cultures, because they avoid a high initial substrate concentration, which imposes osmotic stress and can lead to a so-called overflow metabolism (cells quickly metabolizing a bulk of substrate tend to produce by-products which in turn can acidify the medium and negatively influence cell vitality). A controlled, reoccurring supply of substrate, on the other hand, offers just the right amount of substrate to the cell, minimizing the maximal growth rate but potentially increasing overall biomass production.
While commonly used in larger scale fermentations in bioreactors, fed-batch fermentations in shake flasks are rare due to the lack of technology. When designing experiments for e.g., protein production, it is essential to test different conditions or production strains in smaller (shake flask) scales, using similar feeding profiles as in the final (larger scale) experiments.
Starting a fed-batch process after a certain time requires good knowledge of the process. Have the cells used up most of the substrate after the given time already? Did the biomass increase sufficiently so that the added substrate would not cause osmotic or metabolic stress to the cells? These questions might especially be hard to answer in early shake flask-scale tests that mainly aim to obtain information. Letting the biomass threshold dictate the start of the feeding process makes sure the right time point is chosen without having to know the exact growth curve progression of the respective organism in the respective conditions.
Additionally, biomass-induced fed-batch cultivations of precultures can improve their quality by making sure they are in the same metabolic state when being transferred to the main culture. Read more in our blog post on this topic.
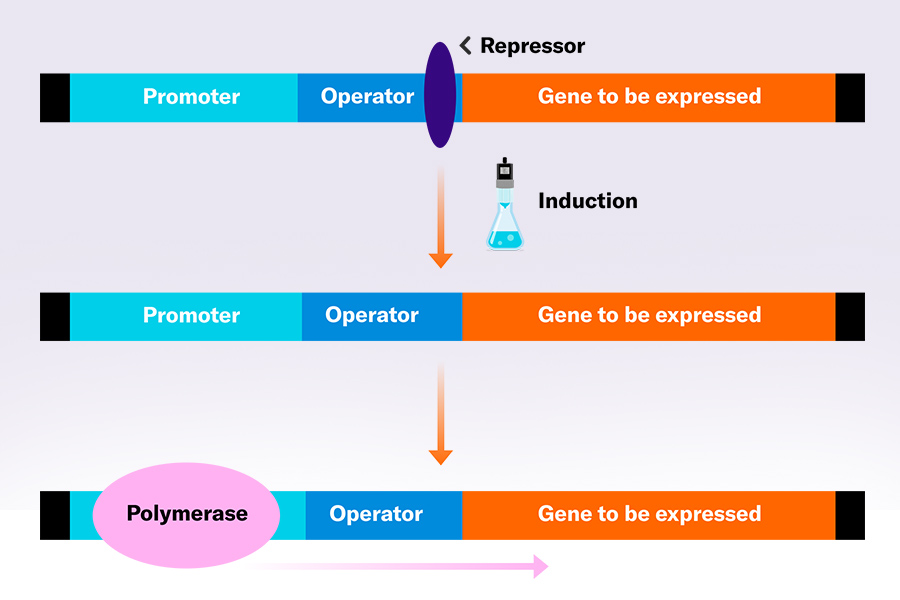
Promotors – the DNA sequences upstream of genes that initiate the genes’ expression – play a huge role in many bioprocesses. When producing recombinant proteins in microbial cells, inducible promotors are often used which allow the cells to maintain in an “expression-off mode” during cell growth, minimizing the additional metabolic burden. This is even more valuable if the expressed protein has toxic effects on the cell. With non-inducible promotors, the selection pressure is in favor of non-expressing mutant cells which would lead to lower overall protein levels. Substances that activate gene expression are called inducers and are small molecules. Depending on the promotor, sugars like IPTG (isopropylthio-β-galactoside), allolactose, arabinose, or alcohols like methanol can serve as inducers. By adjusting the inducer concentration, expression levels can furthermore be controlled. The right inducer concentration or induction time point for individual bioprocesses can be determined via induction screenings – a setup of experiments in which different induction conditions are tested.
However, inducing at the right time point is usually very time-consuming and necesitates intensive sampling and testing. And still, with unexact data points, the ideal induction time point is often missed.
Choosing the right time point for promotor induction is crucial, especially in processes that aim to produce high yields of recombinant proteins. The difficulty here is that the perfect time point is dependent on the cell growth – a parameter that is hard to reproduce and will therefore differ among cultures, even if the same conditions are tried to be maintained. Have the cell growth automatically define the start of induction is therefore a convenient solution for this challenge. Also, when applied in small vessels like shake flasks, numerous different starting points (at different cell growth levels) could be tested in induction screenings to figure out which is the most favorable one for the specific bioprocess.
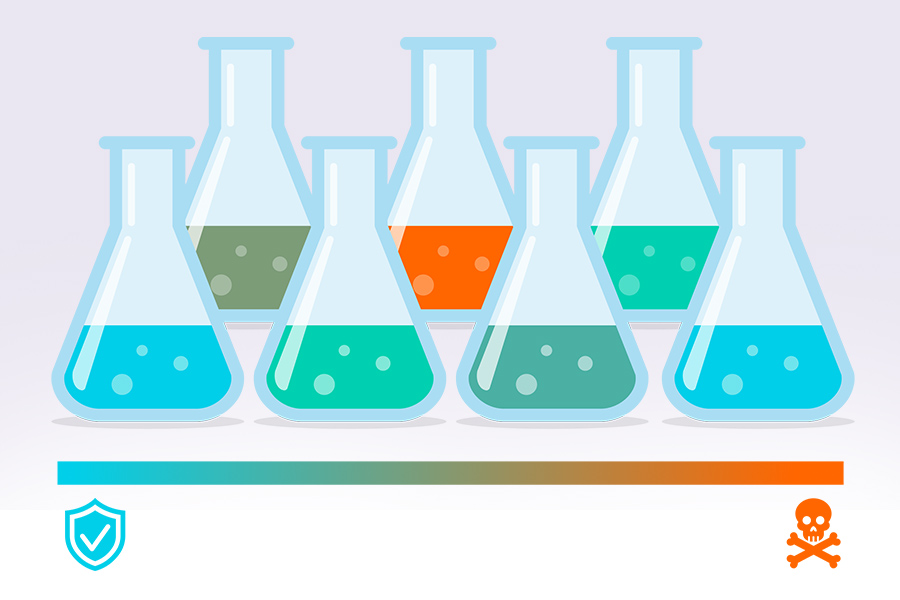
Quite frequently, certain compounds in the medium or metabolic products can have toxic effects on microbial cells. It is often desirable to quantify the toxicity of specific compounds to e.g., estimate effects on the cell’s metabolism, draw conclusions about the best media compositions, or calculate the highest possible yields of a specific product that becomes toxic at certain concentrations. Therefore, tests determining the effect of different compound concentrations on cell growth are a common method applied in laboratories.
Usually, the medium with different concentrations of the respective compound is provided in shake flasks and cell growth is tightly monitored. Naturally, many toxic compounds would prolong the initial lag phase in which no cell growth is visible. However, in many cases the situation might be more complicated and necessitate advanced analysis. If the compound in question has an effect, for example, on proteins that are expressed from genes on plasmids leading to a lower growth rate, the cell might react to this by enhancing the expression level of plasmids to counteract the effect. In these cases, it is beneficial to let the cells grow under the same (media) conditions until a certain growth rate is reached and start feeding the substance only then, observing the immediate substance-induced drop of growth rate. This can offer more in-depth insights into the metabolic effects since effects on protein expression would not be visible so quickly.

In the complex world of bioprocesses there is always room for improvements, even in the first steps, like the inoculation of the medium with cells. It is possible to optimize the inoculum by preparing the cells to the bioprocess conditions, that is: The temperature, shaking speed and most of all, the media compositions. Without the addition of carbon source (yet), cell growth in this preconditioning phase is still prohibited. This slow adaptation to the media compositions with LIS as an automated feeding technique and a preprogrammed shaker has been shown to reduce the lag phase and introduce an additional point of control to your bioprocess, while reducing manual work. The adjusted metabolic state of cells improves the quality of precultures and enhances reproducibility of main cultures. LIS can also be programmed to start, e.g., on weekends, using times that were previously not used effectively, and providing freshly grown cultures on Monday mornings. Learn more by downloading the application note.
Besides the slow adaptation of cells to the medium components by keeping them in the LIS reservoir, automated feeding can also be used for co-cultivations. Two types of cells are sometimes cultivated in the same medium for diverse purposes, e.g., for an improved availability of carbon sources or enzymes. In many cases, the growth behaviour of the two cell types are not identical and it might be useful to start cultivating one (the slower growing) cell type first and only add the second one, when a predefined biomass of the first cell type has been reached.
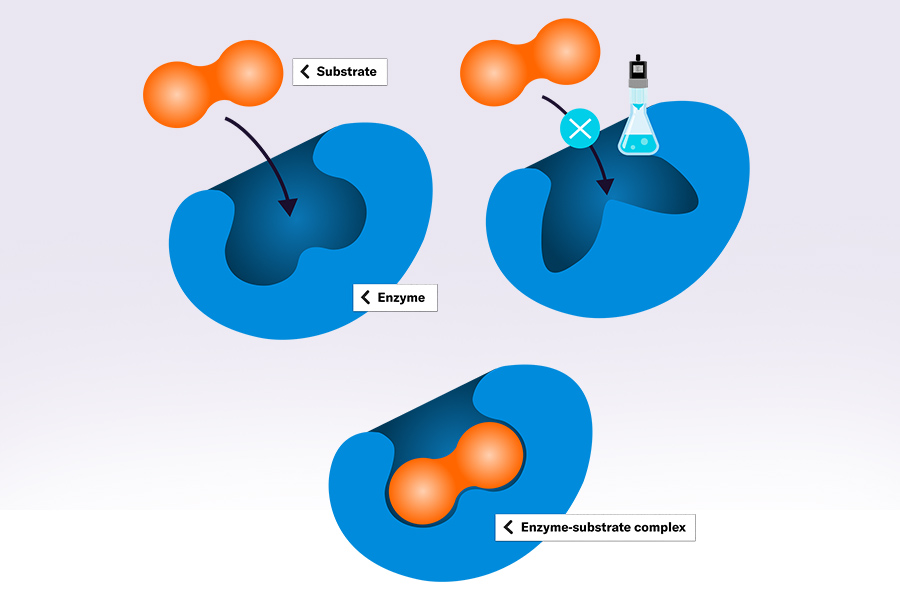
There are many reasons why researchers would want to inhibit specific proteins or enzymes. An enzyme might degrade the desired product, for example, or the experiment aims to determine the specific metabolic situation without a certain enzyme activity. Testing substrates for enzyme inhibiting effects can also serve as pre-clinical drug test and help to understand the detailed mode of action of inhibitors. Different forms of inhibition exist. An inhibitor can bind to the substrate binding site, also called active site, and thereby be in competition with the actual substrate. Or it can bind to a so-called allosteric site, apart from the substrate cavity and change its overall structure, slowing down or eliminating enzyme activity.
Microbial cultures undergo different growth phases that can be clearly distinguished when looking at the biomass concentrations or the resulting growth curves. What is not visible at first sight is that changes do not only occur on a cell growth level but also inside cells. The cells’ metabolic profiles shift in the different phases. Certain enzymes are more or only expressed in certain phases. Connecting the addition of inhibitor with a certain growth rate can therefore be advisable.
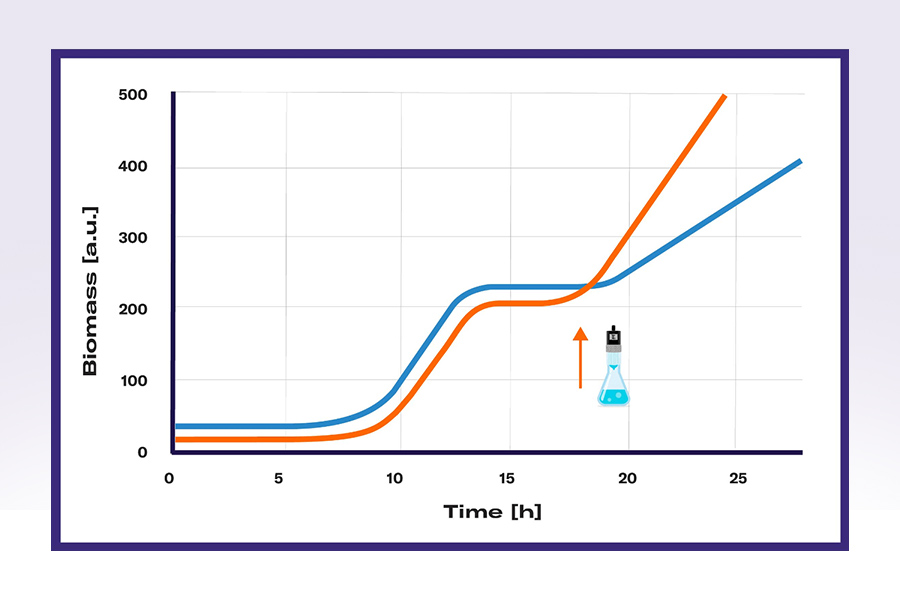
Different organisms and strains will show different growth behaviors and some growth patterns are typical for certain organism groups. A typical growth pattern of yeast is the diauxic shift or diphasic growth form. When plenty of glucose is present in the medium, the yeast cells will use it as its preferred carbon source, resulting not only in rapid growth but also in the formation of ethanol. Once glucose becomes limiting, the cells will shift their substrate use from glucose to ethanol. This shift is characterized by a lag phase (in which the cells initiate the expression of enzymes needed for the metabolism of ethanol). It is followed by a slower growth in which ethanol is aerobically respired. Depending on the industrial purpose, this growth pattern can be accepted or even wanted. However, in certain cases, it is beneficial to circumvent this metabolic shift, e.g., if the desired product is ethanol.
The metabolic shift of yeast cells or other cell specific growth patterns can be manipulated by feeding substrate at the critical time points. Providing more glucose by feeding once a reduced growth rate is detected with yeast cells could, for example, prevent the usage of ethanol and increase overall biomass and ethanol titers. The shift of metabolism has several other cellular effects that might influence bioprocesses, making this application an asset for many purposes.
.png)
Shake flasks are versatile reaction vessels, often used for countless experiments. Ranging from several mL (e.g., 50 mL) till large flasks of up to 5 L, they offer multiple reaction volumes and enable the upscaling of experiments until a certain level. In comparison to very small reaction vessels (test tubes or microtiter plates), shake flasks offer advanced cultivation conditions, including better aeration and mixing of media that are beneficial for the growth of many microorganisms. In comparison to bioreactors, these smaller scale vessels enable low-cost tests due to reduced medium and nutrient quantities used. Because they are easier to set up than bioreactors, the experiments save time and labor costs. Using less space, shake flasks are ideal for the testing of different process parameters or for repeating experiment conditions to prove reproducibility because of a flexible scalability. Many experiments therefore heavily rely on the usage of shake flasks. However, they usually lack the advanced control options that bioreactors can offer.
With technological advances, more and more options are introduced to the world of shake flasks. Having a biomass-based feeding option for this vessel type will be a huge improvement for many applications and a step towards the fully monitored and controlled smart shake flask.
Besides biomass, other parameters can be used to initiate liquid injection, already. With the DOTS platform, advanced bioprocess control options in shake flasks are now a reality.
.png?width=460&height=414&name=Thermometer%20(2).png)
With the DOTS sensors and actuators, shake flasks gain data access and control options and can now compete with advanced bioreactor processes, while staying low-cost, parallelizable, and easy to use.

As the central piece of our DOTS Platform, the MPS enables effortless online monitoring of multiple parameters in shake flasks. Do you need to monitor biomass? Fluorescence? Dissolved Oxygen? Maybe you want it all at once? Do you want to add environmental parameters, like temperature and shaking speed? All this and more is now possible.

The easiest way to measure dissolved oxygen (DO) in shake flasks! By just adding the factory-calibrated and pre-sterilized sensor to the medium and mounting the flask on top of the Multiparameter Sensor, DO can be measured effortlessly, providing high-density and real-time data. When combined with the Liquid Injection System (LIS), DO-based feeding can be realized! Available in different form factors, including DO Pills and DO Nanoparticles.

As an integral part of the DOTS Platform, the Liquid Injection System (LIS) serves as a valuable tool for automated feeding in shake flasks. It opens the door to bioreactor-like bioprocesses within shake flasks, making fed-batch, biomass- or DO-based feeding, or automated promotor induction all attainable through its advanced capabilities.