Data Spotlight
Accuracy of Biomass Measurement Methods
Background
The best method for measuring the biomass of biological cultures has been a subject of ongoing debate. Three primary techniques are commonly used:
1) Optical density (OD), which is widely regarded as the gold standard in many laboratories,
2) Backscatter readings, which are gaining popularity, and
3) Cell dry weight (CDW) determination.
Optical density is favored for its simplicity and quick application, while backscatter readings offer similar benefits, becoming increasingly popular due to their ease of use. On the other hand, CDW determinations, although highly accurate, require multiple steps and significant amounts of medium, making them less practical for routine use.
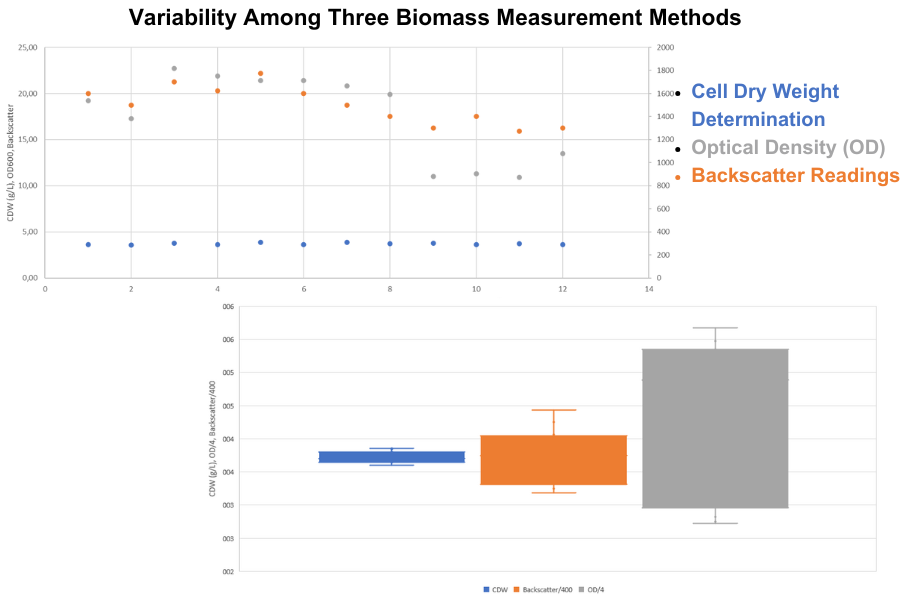
To identify the most reliable technique for measuring biomass, Raul Reveles from Bond Pet Foods conducted tests on six yeast strains, running each test in duplicates using the three different methods. He then calculated the variability between measurements for each method to assess their consistency.
Results
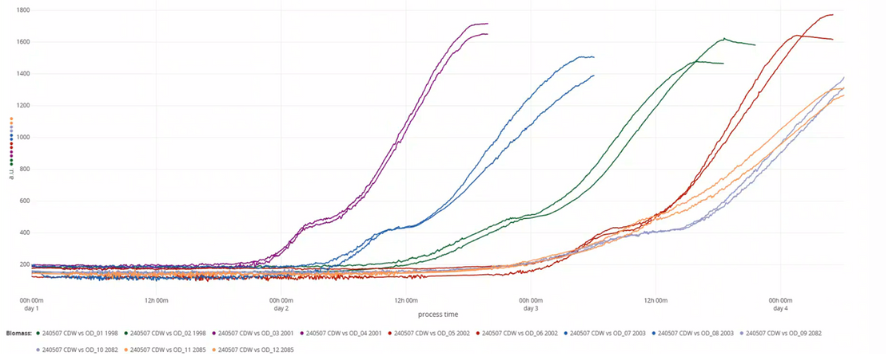
Backscatter readings of six yeast strains, grown in duplicates over four days. Readings were monitored with the DOTS Software.
Materials & Methods
Six yeast strains were grown in duplicate on a defined medium until they reached the stationary phase. The biomass of all strains was expected to be similar. Each culture was then assessed using three different biomass measurement methods. For cell dry weight determination, the cells were harvested, freeze-dried, and weighed. Optical density was measured at 600 nm using a photometer, in which samples were diluted to an OD600 <0.8 to ensure accurate readings. The final backscatter readings of the cultures were automatically recorded through the DOTS Software. All results were visualized and compared for their variability.
Conclusion
The cell dry weight (CDW) determination yielded highly consistent results across all measurements, with very little variation (a coefficient of variation (CV) of just 2.4 %). In contrast, the two optical measurement methods exhibited higher variability, with a CV of 11.1 % for backscatter readings and 26.3 % for OD600 measurements.
This outcome was anticipated, as CDW provides a direct measurement of biomass. However, this method is quite labor-intensive and requires the removal of a significant fraction of cells from the culture, making it unsuitable for some experimental setups.
On the other hand, optical measurement methods are typically much easier to apply. They involve simple sampling, dilution if necessary, and measurement using a photometer (OD) or even just non-invasive, online assessments (backscatter readings). However, these methods can be influenced by factors such as cell shape, size, and medium composition, which reduces their accuracy compared to CDW. Additionally, the need to dilute samples for most OD measurements to avoid reaching the photometer's saturation point can introduce human error, further diminishing accuracy. A comparison of the two optical methods revealed that backscatter readings were notably more consistent than OD600 measurements, with a CV less than half that of OD600. This indicates that backscatter readings may provide a more reliable and accurate means of measurement.
Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)

