How Stopping Your Shaker Affects Your Bioprocess

The Incubator Shaker
Shakers can come in a variety of formats, sizes, and functions. Open-air platform shakers can be placed on a benchtop or inside incubators. Incubator shakers, which might also be called shaking incubators or thermal shakers, fulfill an additional role as an incubator by enclosing the sample and providing temperature control.
Found in almost all biological laboratories, the incubator shaker is a central piece of equipment, designed to provide controlled conditions for the cultivation and growth of microorganisms. At its core, it is equipped with a platform that holds culture vessels such as flasks, and sometimes bottles, tubes or plates containing microbial or cellular samples. The shaking mechanism imparts agitation to the cultures, ensuring uniform distribution of nutrients, gases, and temperature throughout the medium. This dynamic movement promotes optimal growth and productivity in microbial or cell cultures. The incubation aspect of the equipment allows precise control of temperature, creating a stable environment suitable for the specific requirements of the organisms being cultivated. Some advanced models may also feature programmable controls, allowing researchers to customize shaking speed, temperature profiles, and other parameters to meet the unique needs of their experiments.
Shaking It Up!
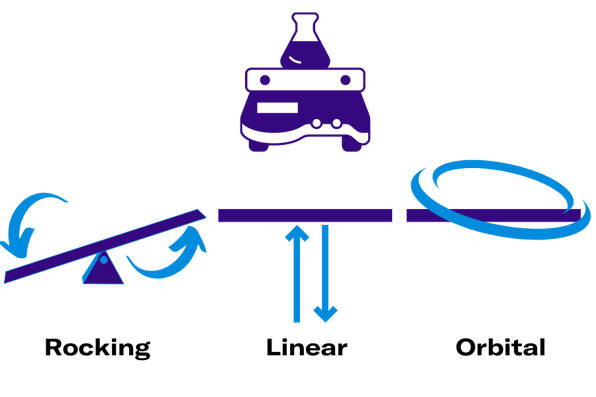
When it comes to the shaking part of the incubator shaker, there are basically three different modes of agitation at our disposal: Rocking, linear shaking, and orbital shaking.
Rocking shakers move in a see-saw motion centered around a pivotal point, creating a gentle wave-like motion. This method is ideal for motion-sensitive procedures such as staining or destaining of gels, DNA extraction, and mixing shear stress-sensitive cell lines.
Linear shakers move back and forth along a one-dimensional plane. While these shaking movements play a minor role in biological experiments, they are still applicable for specific purposes.
On the contrary, orbital shakers are extensively used in laboratories. In this mode, the platform rotates in a circular motion, causing the liquid in a shake flask to swirl in a coherent and mostly in-phase manner, facilitating effective mixing.
In addition to the shaking mode, there are two further factors that characterize the shaking movement; the shaking diameter, or throw, and the shaking speed. Orbital shakers commonly have diameters ranging between 19 and 50 mm, whereas the standard in microbiology ranges between 19 and 25 mm. Larger orbits, especially those exceeding 25 mm, can increase the surface area coverage of samples when operated at speeds of 150 revolutions per minute (rpm) and slower. This becomes particularly advantageous when dealing with delicate cell cultures sensitive to shear stress (the mechanical stress caused by liquid or particle movement). Moreover, in applications involving large-sized vessels such as shake flasks of 2 liters or more, opting for larger orbits may contribute to maintaining a consistent mixing motion, where smaller orbits might fail to do so.
The shaking speed is a parameter that can usually be easily adjusted in most available incubator shakers. In general, higher shaking speeds increase the surface area for gas exchange, providing higher aeration. For more viscous liquids, higher speeds might be necessary to ensure sufficient mixing. However, it’s important to note that higher speeds can also generate shear forces that may negatively impact cells, especially those sensitive to shear stress. Mammalian cells are typically more sensitive, whereas yeast and most bacterial cells are robust and can withstand higher shear forces. Another critical consideration is mixing regularity. Scientists aim to avoid out-of-phase mixing, as it can introduce irregularities that might negatively affect the experiment’s outcome. Additionally, spillage onto the walls and lid of the vessel may increase the risk of contamination.
Continuous Agitation: Important For Optimal And Reproducible Bioprocess Outcomes!
Once the suitable shaking mode, frequency, and diameter have been determined, it is crucial to ensure continuous agitation throughout the fermentation process. Frequent disruptions or extended pauses in shaking can lead to undesirable consequences, including:
- Inadequate nutrient dispersion
- Uneven distribution of temperature, with higher temperatures on the vessel’s edge
- Diminished oxygen availability due to a reduced surface exposure
- Cell sedimentation

And these initial effects can give rise to further detrimental consequences. For instance, an insufficient dispersion of nutrients or other substances may result in unfavorable pH values. Additionally, it has the potential to induce genetic modifications in microorganisms due to selection stress, leading to undesirable behavioral changes. Another example of the negative repercussions of inadequate substrate distribution is the loss of antibiotic selection pressure if this is applied in the respective culture. Antibiotics are frequently introduced into shake flask cultures to uphold selection pressure, ensuring the stability of plasmids. Failure to distribute this selection pressure consistently and uniformly could result in cells losing their plasmids, thereby compromising a crucial characteristic for the fermentation process. And of course, with the implementation of an automated feeding technique, it is especially important to ensure thorough mixing following substance introduction. This precaution is essential to prevent the accumulation of potentially harmful concentrations in the fed liquid.
Furthermore, the impact of reduced oxygen availability on aerobic cultures should not be overlooked. Aerobic cultures, comprising most bioprocessing organisms, rely on oxygen for both cell growth and metabolism. Consequently, the availability of oxygen directly affects cell viability and process titers. Oxygen availability is determined by the oxygen intake rate of the microorganism in question (Oxygen Uptake Rate) and the rate at which oxygen transfers from the gas to the liquid phase (Oxygen Transfer Rate = OTR). Bioprocess conditions, such as higher shaking rates or adjustments to filling volume, can influence this oxygen transfer rate. In many fermentation processes, oxygen serves as a limiting factor, and interruptions in shaking contribute to this limitation.
If you want to learn more about the significance of dissolved oxygen supply and how it can be measured, check out our Dissolved Oxygen Monitoring Application Page!
Many shake flask processes aim to collect data that assists researchers in scaling up their processes to the bioreactor level, aiming for higher product titers. It has been shown in many studies that upscaling can be challenging and that assumptions derived from shake flask data may not seamlessly apply to larger volumes. Successful upscaling necessitates a concerted effort to maintain consistent process conditions across different stages. While some changes are inevitable due to variations in operation modes between shake flasks and bioreactors, certain parameters, such as the availability of dissolved oxygen or the Oxygen Transfer Rate (OTR), play a crucial role in these endeavors and can be calculated using the KLa. Unlike shake flask processes, bioreactor processes are typically not interrupted as frequently, making the maintenance of consistent shake flask agitation vital for more reliable upscaling.

Don’t Forget About Temperature!
Incubator shakers typically maintain a higher internal temperature than the ambient room temperature. Consequently, frequent interruptions involving the stopping and opening of the shaker not only disturb the mixing but also result in frequent temperature drops. While the incubator can readjust the temperature upon closure, this process may incur significant energy consumption. Temperature drops in your fermentation culture – even for limited times – can influence the cell metabolism.
Why Does The Shaker Frequently Stop?
Modern incubator shakers are designed to operate reliably, minimizing interruptions due to malfunctions or technical issues. The majority of shaking interruptions derive from the experimenters themselves. In many laboratories, a single incubator shaker is shared among multiple scientists working on diverse projects. The varying durations and sampling frequencies of these projects result in frequent shaker stoppages with different opening times, as multiple individuals seek access to their flasks at various points. In larger shakers, accessing shake flasks may become more challenging, leading to prolonged interruptions as experimenters remove specific flasks. For those who need to manually sample their culture during the fermentation process, the culture must be taken out of the shaker, potentially transferred under a sterile bench for aseptic conditions, and samples removed before returning the shake flasks. This extended process not only hinders mixing and reduces oxygen supply but also exposes the culture to altered atmospheric conditions, such as temperature or humidity.
How To Avoid Shaking Interruptions
Shaking interruptions can significantly impact your bioprocess, making it crucial to minimize disruptions and reduce shaker opening times. One practical approach is to position the shaker at a convenient operating height with ample space in front for easier access. Many modern shakers automatically halt the shaking movement when the door is opened, expediting the process.
Reducing the number of individuals operating a single incubator shaker can also be beneficial. If the capacity allows, consider reserving the entire shaker for your experiment, minimizing the chances of additional interruptions by others.
While lowering the sampling frequency may not be feasible for many experiments, an effective method to avoid fermentation interruptions is the implementation of automatic sensors. Non-invasive biomass, dissolved oxygen (DO), or pH sensors can eliminate the need for manual sampling. Additionally, modern automated feeding techniques for shake flasks can make manual feeding unnecessary, further automating the shake flask process.
To summarize, here are the top 3 measures to avoid shaking interruptions:
- Convenient positioning: Ensure the shaker is conveniently positioned to enable easy access to shake flasks, reducing door opening time.
- Exclusive booking: If feasible, book the entire shaker for your experiment to minimize additional interruptions by others.
- Automation implementation: Implement automated sampling and feeding techniques to minimize the need for manual sampling and further streamline the shake flask process.
Check out sbi's solutions for automated, online monitoring and feeding with the DOTS Platform!
 Conclusion - Don't Stop Shaking!
Conclusion - Don't Stop Shaking!
Contributing to shake flask challenges, the interruptions stemming from stopped shakers and frequent openings negatively impact process reliability and the ultimate quality of outcomes. Yet, this challenge is actually one that can easily be avoided. By strategically reducing shaker openings through the adoption of automated measurements, sidestepping manual sampling, and carving out dedicated space for your project, the path to a smoother bioprocess becomes clearer. While some measures may present challenges, others are easy enough to implement. Following these strategies not only reduces disruptions but also ensures a smooth and successful journey for your bioprocess, leading to improved outcomes. So, in short: Keep it shaking, keep it happy!



