Overview

Key Facts
Department: CentraleSupélec (Université Paris-Saclay), Chaire de Biotechnologie
Location: Pomacle, France
Employees: 30 (Chaire de Biotechnologie)
Research focus: Modeling of bioprocesses and biomaterials
Organism: Microalgae and yeast (Saccharomyces cerevisiae)
Publication: Cui, N., Pozzobon, V., 2022: Food-grade cultivation of Saccharomyces cerevisiae from potato waste.
LinkedIn: https://fr.linkedin.com/showcase/chaire-biotechnologie-centralesupelec
Creating the Bioindustry of Tomorrow
CentraleSupélec is a French graduate engineering school and part of the Université Paris-Saclay. Its Chaire de Biotechnologie has three areas of expertise, which are:
- Characterization & conversion of lignocellulosics
- Biotransformation
- Downstream processing
Within these areas, a big focus of the group is the modeling of bioprocesses and biomaterials, to optimize their performance. The derived data is a valuable contribution to the successful upscaling of processes for industrial purposes.
One example is the proof of concept of the food-grade cultivation of S. cerevisiae from potato waste. So-called single-cell proteins or microbial proteins can be used as ingredients for protein-rich foods and offer many advantages in comparison to conventional protein sources from crops.
By using agricultural waste as substrate to grow biomass in fermenters, protein production from microorganisms saves resources and is cost-efficient. Additionally, such processes upgrade the food waste, valorizing it in a way in which its nutritional value is preserved. Meal palatability, protein content, and vitamin availability are often improved by the fermentation process with immense benefits for human diet or livestock feed.
The waste from potatoes is one preferred choice as substrate for biomass production because these are the mostly consumed vegetables in developed countries. Potato waste is derived from plenty of sources, e.g., potato peel from the chip-making industry or whole potatoes that are sorted out for aesthetic reasons, and can even be stored for prolonged time periods. Therefore, it is available throughout the year, an important factor when considering industry-scale production of protein from this source.
Victor Pozzobon and his coworkers deconstructed the potato waste by acid hydrolysis, making the incorporated sugars available to cells. They then tested different cultivation conditions in shake flasks to provide data on the feasibility of single-cell protein production with S. cerevisiae on potato waste and model the presumed productivity of such a process in a large-scale bioreactor.
Challenges of Feeding Yeast with Potato Waste
Necessitates hydrolysis to make sugars accessible
Heterologous substrate - contains sugars but also other components
Growth inhibitors reduce the growth rate of yeast
Finding the right feeding balance is crucial for optimal growth
The hydrolysate of potato waste contains valuable sugars that the yeast S. cerevisiae can use for cell growth and protein production. However, it also contains several growth inhibitors, like furfural, acetic acids, formic acids, and others. When accumulated, these inhibitors have a detrimental effect on cell growth and biomass production suffers. Batch cultures therefore need a careful consideration of potato waste treatment and the supplementation of this hydrolysate to the medium – too much will contract cell growth because of growth inhibitors but too less limits cell growth as well, because less sugar will be available.
In the batch process, the substrate was completely supplied to the culture from the beginning. In a second approach, a fed-batch process, using the Liquid Injection System (LIS) was implemented. For this Victor Pozzobon and his coworkers used a shake flask with two necks (38 mm). The second (lateral) neck was used to insert a needle through a cotton cap for air injection to avoid oxygen limitation. The LIS setup was adjusted onto the primary, straight neck. The prepared concentrated hydrolysate (20 mL) was entered into the sterile LIS cartridge which was then connected with the LIS Drive. A multi shot feeding profile was adjusted with the software to allow substrate feeding in a fed-batch manner. 20 mL of hydrolysate were fed in 4 steps equally (5 mL each) over a cultivation time of 50 hours. Cell growth was monitored online and continuously with the Cell Growth Quantifier (CGQ) and online OD600 values were derived from a two-point calibration.

Results
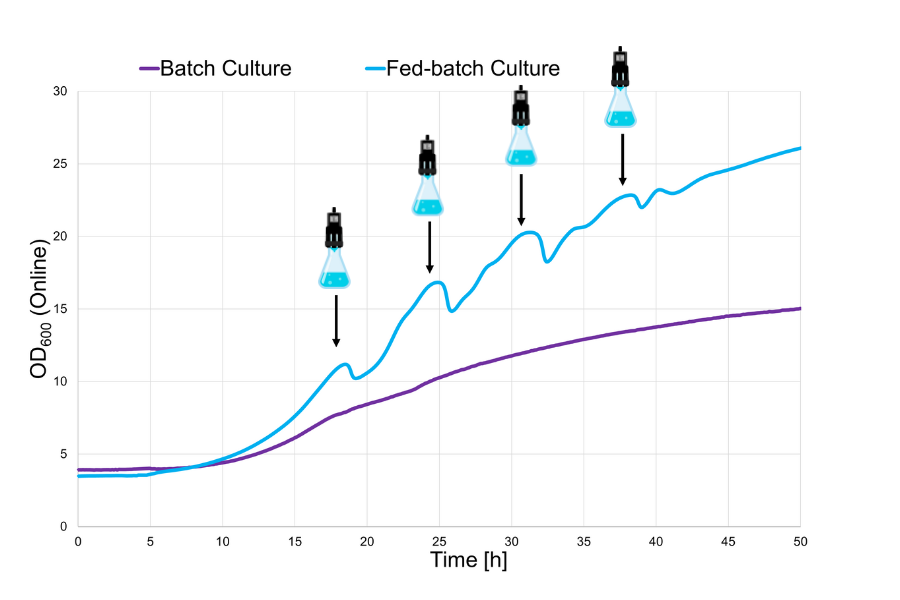
In the batch culture, the complete, concentrated hydrolysate was supplied to the culture from the very beginning, adding higher sugar concentrations to the medium. Also, higher amounts of unwanted substances that negatively influence yeast cell growth were present.
The fed-batch process, realized with sbi’s LIS setup, reduced the initial concentration of growth inhibitors in the medium. This concentration slowly increased with each feeding step, so that the higher cell densities that were reached in between feeding steps could tolerate the overall composition more easily. The cells could therefore utilize the sugars of the hydrolysate in the same, biomass supporting way, whereas the effect of the growth inhibitors was reduced, compared to the batch procedure. This became apparent in the overall growth behavior of the yeast. The fed-batch culture reached a final OD600 of 26 (in a final volume of 70 mL), whereas the maximum final OD600 of the batch culture was only 15 (in 50 mL).
The Conclusion
A fed-batch process improves the growth of yeast cells on concentrated potato feed, because toxic effects of this substrate can be contained. With the LIS, it was possible to show this on a shake flask level, imitating bioreactor-scale runs. This allows for the collection of valuable data enabling the modeling of industrial bioprocesses, such as the production of single-cell protein from potato waste.
Want Results Like These?
We will work with you on a solution that works best for your application.
Testimonial
"With the LIS, I was able to recreate typical bioreactor processes, like fed-batch cultivations, in shake flasks. This allowed me to collect valuable data enabling the modeling of industrial bioprocesses, such as the production of single-cell protein from potato waste."
- Dr. Victor Pozzobon, Chaire de Biotechnologie, CentraleSupélec
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)