Data Spotlight
Disruption-free, On-the-fly Monitoring of Bacterial Oxygen Use for Assessing Cell Fitness
Background
The Multiparameter Sensor (MPS), when used in combination with DO Sensor Pills, enables simple and continuous monitoring of dissolved oxygen levels in shake flask cultures — without disrupting the experiment.
Oxygen consumption serves as a sensitive and dynamic indicator of bacterial growth and fitness. The faster bacteria grow and metabolize, the more oxygen they consume. Tracking DO over time therefore provides valuable insight into the physiological state of the culture.
In this study, E. coli cultures were transformed using the novel BPP Bioportide™ method and were compared to cultures transformed via conventional heat shock. Unlike heat shock, BPP Bioportide™ allows for nucleic acid delivery without stressing the cells. This gentler transformation process is expected to better support growth and productivity.
The resulting oxygen consumption profiles clearly highlight differences in cell fitness between the two transformation methods — with faster oxygen uptake reflecting a shortened lag phase and hence improved vitality in Bioportide-treated cultures.
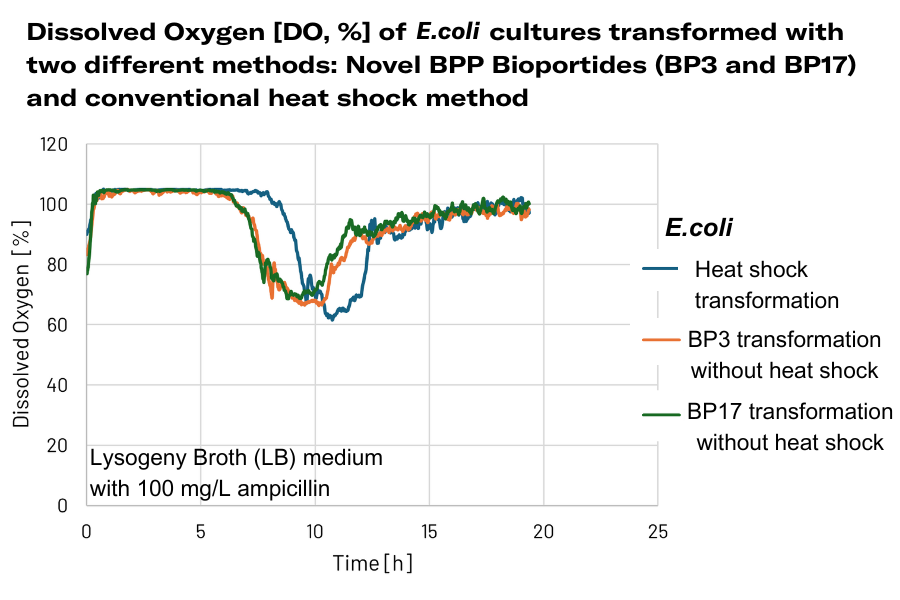
The BPP Bioportide™ treated cells (BP3 and BP17) start taking up oxygen faster than the calcium-competent heat shocked cells.
Materials & Methods
E. coli cells were either prepared as calcium-competent and transformed with the target DNA using the conventional method, which included a heat shock step. In contrast, cells transformed with BPP Bioportide™ did not undergo heat shock and were instead directly streaked onto plates. By avoiding the heat shock step, improved cell viability was anticipated.
Following transformation, all cultures were grown under identical conditions: Shake flasks (500 mL Erlenmeyer flask with additional neck on the side for optimal oxygen supply) filled with 50 mL Luria-Bertani (LB) medium that contained 100 mg/L ampicillin to maintain selection pressure. The medium was inoculated to an initial OD600 of 0.02, and incubated at 37 °C, 300 rpm, for approximately 19 hours.
DOTS DO Sensor Pills were added to each flask, and the flasks were mounted on the Multiparameter Sensor (MPS). Dissolved oxygen levels were continuously monitored throughout the cultivation and recorded using DOTS Software.
Conclusion
The early phase of culture growth is particularly critical for assessing cell fitness, as it reflects the cells’ recovery and adaptation following transformation. These subtle dynamics can only be accurately observed in liquid cultures, where continuous monitoring is possible — unlike endpoint assessments on solid media.
During this early stage, biomass measurements often lack the sensitivity to capture small but biologically relevant differences in growth behavior. In contrast, dissolved oxygen is a highly responsive parameter, capable of detecting changes in metabolic activity that precede visible biomass accumulation. In this study, fast-growing E. coli cells were used, which typically are inoculated at low initial cell densities. Under these conditions, biomass alone may fail to reveal subtle differences in cellular stress and the impact of the transformation method is harder to distinguish. Therefore oxygen consumption was the prefered indicator for evaluating cell fitness and vitality. The data showed that both replicates that were transformed with the novel BPP Bioportide™ method used oxygen faster (already after approx. 6.8 hours of cultivation), whereas the conventionally transformed cells (which were heat shocked) started to consume oxygen only after 8.3 hours. This delayed oxygen drop indicates that the conventionally transformed cells experienced a prolonged lag phase (approx. 1.5 hours longer than the Bioportide-transformed cells) which is a strong indication for compromised cell fitness.
Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)

