.png?width=2000&height=714&name=Pichia%20pastoris%20-%20plate%20with%20cells%20(1).png)
Pichia pastoris
Pichia pastoris is a methylotrophic yeast species (it can use methanol as a source for energy) that has become a popular protein expression system. Several advantages make it an ideal host for protein expression, e.g.;
- The production of large amounts of recombinant protein
- Post-translational modifications of proteins
- The secretion of proteins into the culture medium
Pichia can be grown in minimal media to high cell densities and is easily genetically manipulated, making it an economical choice for protein expression. Overall, Pichia pastoris is a versatile, efficient, and reliable protein expression system.
Strain Screening
BRAIN Biotech is a leading company in the research, development and production of specialty enzymes with a focus on the food and life science industries. In addition, the company develops microbial production organisms and scalable bioprocesses for the economic production of specialty enzymes and other proteins. Customized innovative biological solutions for more sustainable products and processes round off the portfolio. In this study, scientists from BRAIN Biotech aimed to produce brazzein, a sweet protein (up to 2,000 times sweeter than sugar) with strong potential as a natural, low-calorie sweetener and promising ingredient for the food industry.
Naturally, BRAIN Biotech aimed to maximize protein yields by optimizing production conditions early on and—most importantly—identifying the most promising production strain. To reduce costs, strain selection was carried out at smaller scales. Microtiter Plates (MTPs), which enable high-throughput testing with minimal volumes, are well-suited for initial screenings. However, MTPs have limitations: they often fail to replicate the complex conditions of larger bioreactors. As a result, strains that perform well in MTPs may not exhibit the same productivity when scaled up, potentially leading to unexpected challenges in later production phases.
In this project, the team aimed to achieve recombinant production of brazzein using the yeast Pichia pastoris, testing various strains to identify the most effective producers.
To ensure the overall process remains economically viable, it is critical that strain selection is both robust and reliable. The choice of the production strain significantly impacts success in downstream processes. Therefore, careful planning and thorough evaluation of the experimental design are essential when selecting the optimal production strain.
Curious to learn more about BRAIN Biotech? Visit their website here.
From the MTP screenings, three strains that produced comparable levels of brazzein were selected for further testing. However, once cultivated in bioreactors, their performance deviated significantly from the values found in the MTP - highlighting the challenges of translating small-scale results to larger production environments.
![volumetric yield Brazzein [μgmL] (in % based on 3D7 as 100%)](https://www.scientificbio.com/hs-fs/hubfs/volumetric%20yield%20Brazzein%20%5B%CE%BCgmL%5D%20(in%20%25%20based%20on%203D7%20as%20100%25).jpg?width=713&height=475&name=volumetric%20yield%20Brazzein%20%5B%CE%BCgmL%5D%20(in%20%25%20based%20on%203D7%20as%20100%25).jpg)
The strain BRZ5-5 75-3 emerged as the weakest performer in the bioreactor run, despite having been the top performer in MTP results. It achieved only about 30% of the expected output. This strain contains fewer gene copies, distributed across the genome, and appeared highly sensitive to methanol induction, possibly leading to instability in expression.
The 3D7Δrec strain delivered moderate yields (50–70%). Interestingly, although it is genetically more stable—unable to lose gene copies—it did not outperform its parent strain as anticipated.
The 3D7 strain ultimately demonstrated the highest productivity in the bioreactor run. Its yield was normalized to 100% to enable comparison with the other strains.
These discrepancies between MTP and bioreactor performance suggest that strain selection based solely on MTP results can be misleading.
A likely contributor to this mismatch is the difference in feeding strategies between the two systems, which may influence strain behavior and protein expression under scale-up conditions.
Different Feeding Strategies in MTP vs. Bioreactor Runs
MTPs:
In MTPs, methanol (MeOH) was first added manually after 72 hours of cultivation. Subsequent additions were made every 8 to 24 hours, targeting a final MeOH concentration of approximately 0.5% in the culture. However, this bioprocess was neither monitored nor optimized based on cell growth or metabolic activity.
Bioreactor:
In the bioreactor, cultivation began with a batch phase using 40 g/L glycerol for the first 16–18 hours. This was followed by an exponential glycerol feed over 3.5 hours to maximize biomass accumulation. Subsequently, a constant methanol (MeOH) feed was applied for 4 hours, followed by an exponential MeOH feed lasting 90 hours. These MeOH feeding phases were designed to induce and sustain protein production, ultimately aiming for high product yields.
Alternative feeding strategies were employed because advanced methods such as constant or exponential feeding could not be implemented in MTPs. This limitation is primarily due to the low level of automation available without the use of costly robotic systems. Additionally, the small working volumes in MTPs further restrict the feasibility of sophisticated feeding approaches.
These advanced feeding strategies, however, were applied in bioreactor runs to achieve higher biomass concentrations and ultimately enhance product yields. As a result of the differing feeding strategies used in the MTP and bioreactor experiments, Pichia pastoris cells were exposed to varying MeOH concentrations in each setup.
Ideally, screening conditions should closely mimic those of later production, and bioreactor experiments would provide the most realistic data. However, the high cost and low throughput of bioreactor runs make them impractical for early-stage screening. As a result, researchers must strike a balance between experimental realism and efficiency when selecting production strains.
DO-based Feeding in Shake Flasks Fills the Gap
Parameter-based feeding in shake flasks offers extensive control over bioprocesses in this medium-throughput format. By applying various feeding strategies—ranging from constant and exponential to multi-step profiles—and initiating them based on parameters such as biomass, fluorescence, pH (upcoming), or dissolved oxygen (DO), processes can be tightly regulated and a wide range of conditions simulated. This enables shake flask experiments to closely mimic bioreactor runs, enhancing reproducibility across scales and supporting a more reliable and efficient scale-up.
The Technology
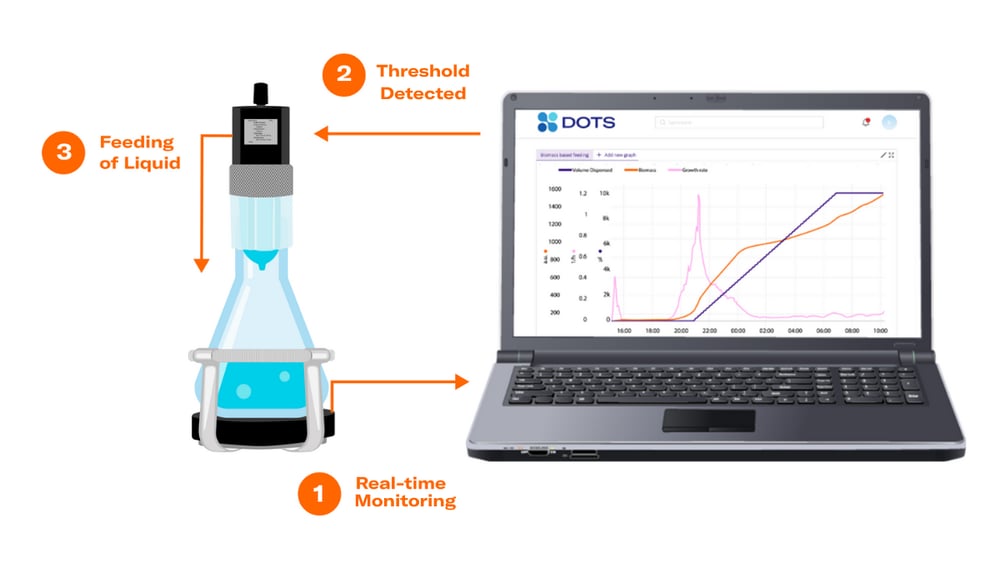
The Multiparameter Sensor (MPS) senses a variety of parameters, such as dissolved oxygen (DO) in real-time (1). The DOTS Software collects and visualizes the data and detects the pre-set threshold (2). Once the threshold is detected, the Liquid Injection System (LIS) starts feeding according to the preset feeding profile.
DO-based Feeding of 20% Methanol Showed Varying MeOH Sensitivities of the Strains
Scientists at BRAIN Biotech integrated the DOTS Platform into their shake flask experiments to monitor biomass and dissolved oxygen (DO) in real time, and to enable DO-based methanol feeding.
The graphs below show two representative shake flask cultivations using strains BRZ5-5 75-3 and 3D7. The Multiparameter Sensor (MPS) was placed beneath the shake flasks, continuously recording biomass (dark blue and dark purple) and DO levels (light blue and light purple). Meanwhile, the Liquid Injection System (LIS) was mounted on top of the flasks, loaded with 20% methanol as the feed solution.
Using the DOTS Software, a DO-based feeding profile was configured. After a batch phase in which cells grew on 10g/l glycerol medium (without additional feeding), a methanol pulse phase was started. In this, methanol feeding with the LIS was triggered once the DO level was higher than 30%, indicating that the previously added methanol had been metabolized by the cells. This setup ensured precise, demand-driven methanol feeding aligned with the metabolic activity of the culture.

Biomass (dark), dissolved oxygen (DO, light), and dispensed volume of 20% MeOH (medium) of P. pastoris strains 3D7 (purple) and BRZ5-5-3 (blue). After an initial batch phase with 10 g/l glycerol in the medium, the MeOH dispension was set to start depending on the DO level. Only when DO recovered to a level above 30%, more MeOH was dispensed. Filling volume was 10% at the beginning and shaking frequency 250 rpm. The temperature was maintained at 30°C.
A comparison of the two strains reveals distinct differences in methanol metabolism.
Strain BRZ5-5 75-3 appears to be more sensitive to methanol toxicity than strain 3D7, and at certain time points, seems to halt methanol metabolism altogether. This is evident from the dissolved oxygen (DO) profile: In the first half of the cultivation, DO levels in BRZ5-5 75-3 show progressively less depletion after each methanol addition—indicating reduced oxygen consumption. At a cultivation time point of 4 days onward, the DO no longer decreases following methanol pulses but instead rises continuously, even as the methanol feed rate increases. This suggests that the cells are no longer metabolizing the added methanol effectively.
In contrast, the DO profile of strain 3D7 shows consistent and immediate oxygen depletion following each methanol pulse, indicating consistent metabolic activity. This suggests that 3D7 is more tolerant to the methanol concentrations introduced during the experiment compared to strain BRZ5-5 75-3.
This difference in methanol tolerance may explain why BRZ5-5 75-3 performed significantly better in MTP experiments than in bioreactors. In bioreactor setups, methanol was likely present at higher concentrations due to continuous feeding, while in MTPs, methanol was added only once as a batch at the beginning. The reduced methanol exposure in MTPs may have favored the metabolic performance of BRZ5-5 75-3.
Mimicking Bioreactor Conditions in Shake Flasks: Combining Glycerol Fed-batch and DO-triggered Methanol Induction
This experiment was designed to closely replicate a bioreactor run. To achieve this, both key phases—the glycerol fed-batch phase and the methanol induction phase—were simulated using the LIS.
The process for all three strains (3D7, 3D7Δrec, BRZ5-5 75-3) began with an initial batch phase, where the medium contained 10 g/L glycerol. This was followed by a fed-batch phase in which a 50% glycerol solution was added. The feeding profile was controlled via the DOTS software, triggering feed events when the dissolved oxygen (DO) levels recovered. This allowed precise substrate delivery tailored to the organism’s metabolic needs, ultimately maximizing biomass production. This phase lasted 50 hours.
After the glycerol phase, the LIS was modified to deliver a 20% methanol solution. For the next 119 hours, methanol was added using a DO-triggered shot strategy—each pulse was administered after the DO level had recovered, ensuring a controlled supply that supported optimal product formation.
This approach allowed the shake flask process to approximate key features of the bioreactor run, including an initial glycerol feeding phase for biomass accumulation and a subsequent methanol induction phase to support protein expression.

The biomass, DO, and feeding profiles of the three strains were generally similar, yet they also offered valuable insights into the metabolic state of the cells. All profiles indicated an initial adaptation phase to the methanol carbon source at the beginning of the methanol feeding on day 3, due to the switch to different metabolic pathways for methanol consumption, common across all strains. During this period, methanol was not metabolized efficiently, as evidenced by elevated DO levels that remained high initially—suggesting minimal metabolic activity. As methanol accumulated, subsequent DO depletion occurred more slowly than expected, likely due to cellular stress or delayed recovery as the cells adjusted to the new substrate.
After completing the adaptation process, all strains efficiently metabolized methanol—except for the 3D7Δrec strain, which showed an abrupt stop in methanol metabolism by the sixth day of fermentation.
The visible jumps in the biomass profile can be explained by sample collection.
Comparison of Results Between the Three Systems: MTP, Shake Flasks, Bioreactors
An analysis of the volumetric brazzein yields across the three strains and vessel types—MTPs, shake flasks, and bioreactors—revealed significant differences in strain performance depending on the cultivation system. While overall trends in shake flasks closely mirrored those in bioreactor runs, yields in MTPs diverged notably. Specifically, strain BRZ5-5 75-3 seemed to outperform the reference strain 3D7 in MTPs, achieving approximately 30% higher product yield. However, in the bioreactor, this relationship was reversed: BRZ5-5 75-3 produced around 40% less brazzein than 3D7.
These findings highlight that, while MTPs are valuable for early-stage screening, their predictive power for large-scale performance is limited. In contrast, modern shake flasks—when operated under conditions that closely simulate bioreactor parameters—offer a more reliable and still cost-effective platform for evaluating strain behavior in scaled-up processes.
![volumetric yield Brazzein [μgmL] (in % based on 3D7 as 100%) (2)](https://www.scientificbio.com/hs-fs/hubfs/volumetric%20yield%20Brazzein%20%5B%CE%BCgmL%5D%20(in%20%25%20based%20on%203D7%20as%20100%25)%20(2).jpg?width=900&height=600&name=volumetric%20yield%20Brazzein%20%5B%CE%BCgmL%5D%20(in%20%25%20based%20on%203D7%20as%20100%25)%20(2).jpg)
With advanced control technologies such as DO-based substrate feeding, shake flasks can increasingly replicate key aspects of bioreactor environments—an essential factor for generating reproducible and meaningful experimental results. Thanks to their medium-throughput capacity and enhanced process control, shake flasks represent a superior alternative to MTPs for strain screening experiments with fed-batch or DO-based feeding, particularly when the goal is to identify candidates that will perform reliably in bioreactor-scale production.
.png?width=100&height=51&name=sbi%20logo%20(npws).png)
Solutions for Smart Strain Selection in Shake Flasks
Shake flasks fill the gap between MTPs and bioreactors. The DOTS Platform was designed to empower researchers to conduct bioreactor-like experiments on a shake flask level, leading to enhanced bioprocess control, improved insights, and cost-efficiency in bioprocess development.
Modern Shake Flask Technologies Improve Strain Screening Processes
With features like DO-based feeding, modern shake flasks make it easier to simulate bioreactor conditions during early-stage strain screening, improving the reliability - and scalability- of results. This can significantly reduce the number of costly and time-consuming bioreactor runs, as reliable results are generated in this medium-throughput system. Monitoring strain-specific responses—such as methanol metabolism—in parallel also helps streamline and speed up process development.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)

Want Results Like These?
We will work with you on a solution that works best for your application.
