Data Spotlight
Validation of Online DO and Biomass Monitoring in CHO Cell Cultures: Strong Correlation and High Reproducibility
Background
Chinese Hamster Ovary (CHO) cells are widely used in biotechnological and pharmaceutical research, particularly for the production of therapeutic proteins. A common method to determine cell growth is to measure biomass through offline sampling techniques such as dry weight analysis or optical density measurements. However, these methods are invasive and require frequent sampling, which increases the risk of contamination and disrupts the cultivation process. This is especially critical when working with slowly growing or sensitive cell cultures, where maintaining sterile conditions is essential. Therefore, process automation and non-invasive monitoring methods are highly desirable.
A working group at Hochschule Bielefeld (HSBI) implemented sbi's Multiparameter Sensor (MPS) system to enable non-invasive, continuous monitoring of biomass in CHOs cell cultures. The sensor data was compared with conventional offline measurements obtained from cell samples taken at defined intervals during cultivation.
In addition to biomass, dissolved oxygen (DO) levels were monitored throughout the experiment to evaluate oxygen consumption and metabolic activity of the cell cultures.
Results

Biomass (purple) and dissolved oxygen (blue) data of a CHO cell cultivation recorded online with the Multiparameter sensor. Offline cell counts are depicted in orange.
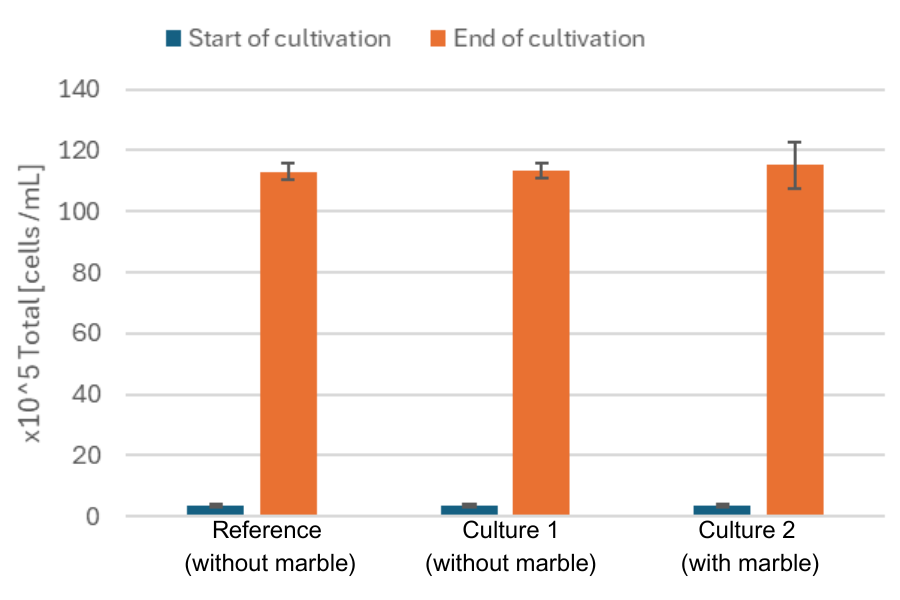
The DO marble does not influence cell count.
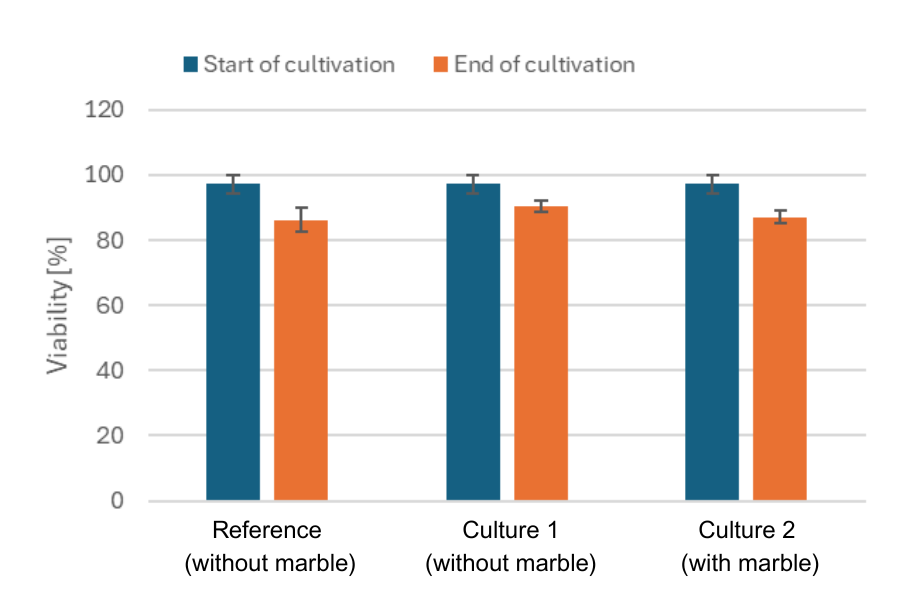
The DO marble does not influence cell viability.
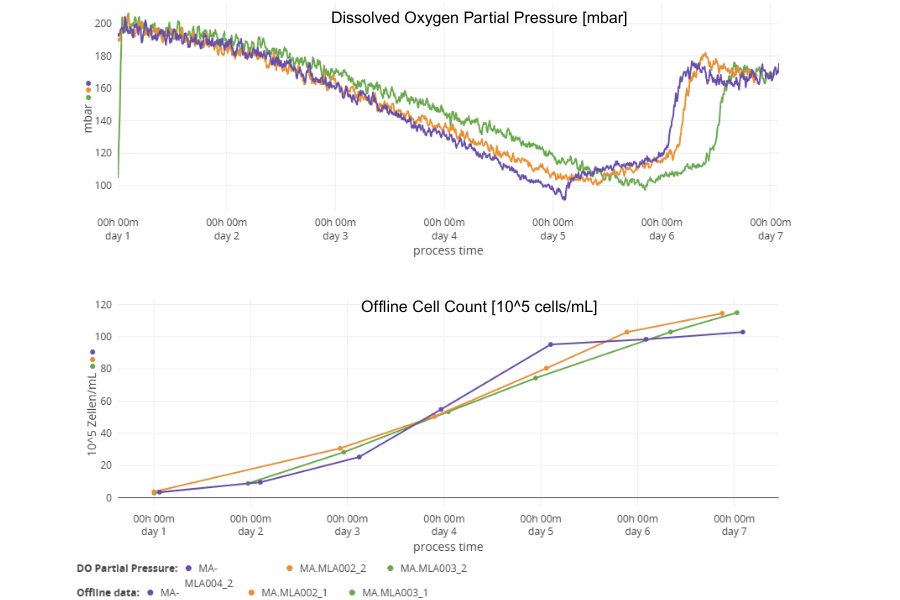
Dissolved oxygen monitoring and offline cell counts of three different cultures. The DO data correlates well with the offline biomass but furthermore shows even subtle differences in cell metabolism.
Materials & Methods
CHO cells were cultivated in 250 mL Corning shake flasks with a 30% medium volume. The cultures were maintained at 37 °C and agitated at 140 rpm for a period of seven days. For online monitoring, a Multiparameter Sensor (MPS) was positioned beneath each flask, and a DO Sensor Marble was added to enable continuous dissolved oxygen measurement. The MPS collected real-time data on both biomass and dissolved oxygen levels throughout the entire cultivation period.
In parallel, daily manual sampling was performed in a seperate flask to assess cell growth offline using the Cedex Bio Analyzer (Roche). To evaluate any potential influence of the DO Sensor Marble on cell growth and viability, cell count and viability were determined at the beginning and end of cultivation for three conditions: the culture with the marble, the culture without the marble, and a reference culture that was used for offline sampling (without marble and MPS).
Conclusion
In conclusion, the data demonstrated a strong correlation between the offline cell counts obtained using the Cedex Bio Analyzer and the online biomass measurements collected via the Multiparameter Sensor, confirming the reliability of the MPS data. Additionally, the DO data recorded by the MPS aligned well with the observed growth phases, further supporting the accuracy of the online monitoring system. Furthermore, the DO data from three different CHO cell cultivations revealed subtle differences that are not detectable with offline biomass measurements, offering deeper insights into the process. Importantly, the presence of the DO Sensor Marble had no detectable impact on cell viability or cell density, indicating that the sensor setup is non-invasive and well-suited for long-term monitoring of CHO cell cultures.
This research was conducted by:
M. Lauer and Prof. Dr. D. Lütkemeyer

Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
