Data Spotlight
Optimized Bioreactor Cultivation of Penicillium sp. for Reproducible Production of an Intracellular Protease Inhibitor
Background
Penicillium sp. produces a promising intracellular protease inhibitor with potential therapeutic application against Human African Trypanosomiasis (HAT). For optimal production, cultivation in pellet form is preferred over free mycelia due to improved process reproducibility and intracellular metabolite retention. In this study, Penicillium sp. was cultured under different conditions to determine optimal medium formulations for reliable pellet formation that lead to the highest space-time yield of the protease inhibitor.
This study evaluated how the spore concentration in the inoculum and the addition of CaCl2 and Pluronic F68 affected the growth of Penicillium sp. and the formation of the protease inhibitor. Determination of biomass production and microscopic examination of thin-sliced pellets were carried out. Both the quantity of the substance produced, and the reproducibility of the process were considered when optimizing fungal cultivation, and a toxicity test was performed to evaluate the potential of the protease inhibitor as a drug candidate.

Growth profile of Penicillium sp. with spore concentration of 4.41×10⁵ spores/L. Real time biomass measurements taken with the CGQ BioR correlated well with offline values.
Solid line: pH value; Solid square: glucose conc.; Hollow square: maltose conc.; Dotted line: biomass conc. from backscatter sensor; Solid circle: bio dry mass (BDM) bdm conc.
Results
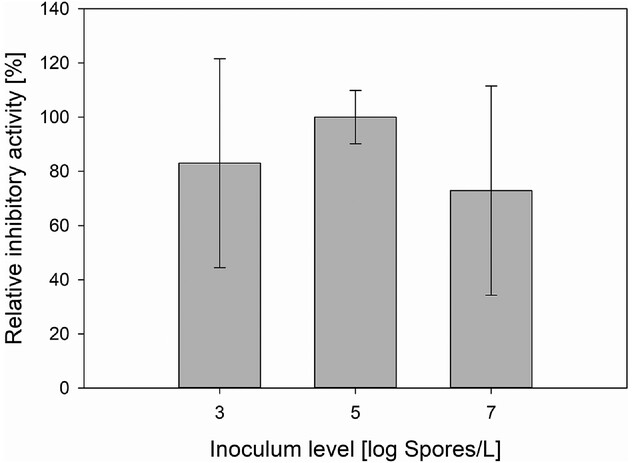
Spore concentrations had little effect on final biomass concentration and growth rate but heavily influenced the duration of the lag phase, the morphology of formed pellets, pellet diameter, and yield of the protease inhibitor. In this specific use-case, the highest space-time yield was achieved with a spore concentration of 4.41×10⁵ Sp/L. While this concentration led to a slightly longer lag phase than a higher starting spore concentration, it exhibited a 27% higher yield of protease inhibitor and the best reproducibility.
- High spore concentration (10⁷ Sp/L) shortened the lag phase (∼30 h) but reduced overall yield and reproducibility. It also resulted in smaller, irregular, “hairy” pellets with dispersed hyphae.
- Low spore concentration (10³ Sp/L) extended the lag phase (∼60 h) and led to hollow, lysed pellets.
- The optimal spore level (10⁵ Sp/L) had a slightly extended lag phase (~45 h) and produced compact, dense pellets with intact cores—ideal for intracellular metabolite accumulation.
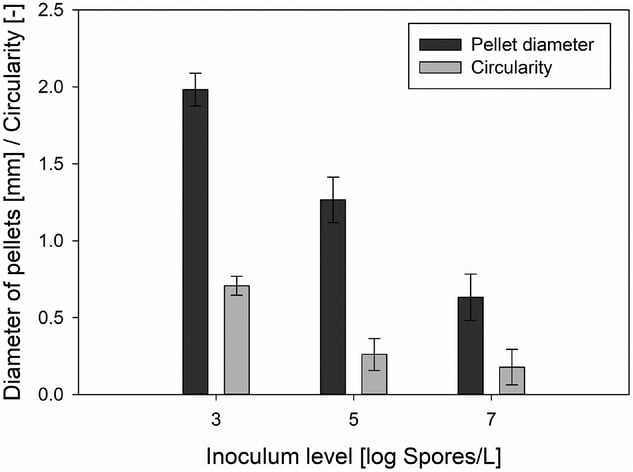
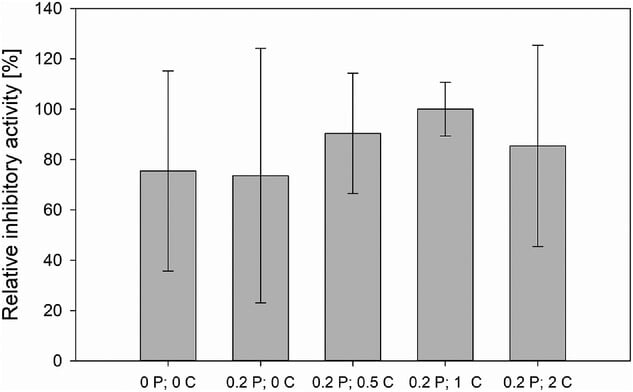
The addition of CaCl2 served to shorten the lag phase by enhancing spore aggregation processes, leading to faster pellet development. No significant shortening of the lag phase was observed when increasing the concentration of CaCl2 from 0.5 g/L to 2.0 g/L, so the optimal concentration (1 g/L) was determined by looking at the combined effect of both CaCl2 and Pluronic F68 during cultivation.
Pluronic F68 reduced the shear stress on forming pellets by stabilizing air bubbles in the culture medium. This slowed the exponential growth phase since the growing pellets retained their shape better with decreased shear stress. As a result, fewer hyphae were shed from the pellets and fewer clumps were formed in the cultivation medium from these hyphae. This effect was mostly insignificant when paired with the effects of CaCl2, emphasizing the importance of CaCl2 in the aggregation process.
- CaCl₂ (1.0 g/L): Accelerated pellet formation during the lag phase by supporting early spore aggregation.
- Pluronic F68 (0.2%): Preserved pellet structure during the exponential phase by reducing hydrodynamic shear stress.
Materials & Methods
Cultivation Setup: Penicillium sp. was grown in a 2L glass bioreactor using YMG medium with varied concentrations of CaCl₂ (0–2.0 g/L), Pluronic F68 (0–0.2% w/v), and spore inoculum levels (10³–10⁷ spores/L). Duration of each cultivation varied based on experimental conditions, with all cultivations considered “finished” with oxygen recovery, or when the aeration decreased back to 1 L/min.
Monitoring: Biomass concentration was assessed online (CGQ BioR backscatter sensors) and offline (gravimetric method). Aeration of the medium was regulated to maintain a dissolved oxygen (DO) saturation of 30%: When the DO dropped below this limit, atmospheric air flow was increased and, if insufficient, was supplemented with pure oxygen.
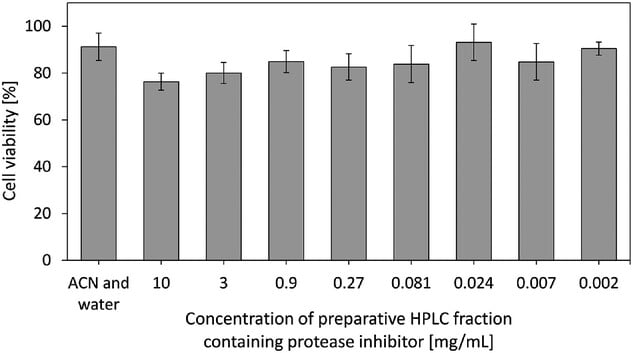
Inhibitor Extraction: Intracellular protease inhibitor was extracted post-cultivation and isolated via HPLC.
Assays: Protease inhibition was measured using a rhodesain enzyme assay. Cytotoxicity was tested on human peripheral blood mononuclear cells (PBMCs) via resazurin assay. Pellet morphology was analyzed by microscopy.
Conclusion
In this study, the combination of 4.41×10⁵ spores/L, 1.0 g/L CaCl₂, and 0.2% Pluronic F68 provided optimal and reproducible conditions for the bioreactor-based production of the non-cytotoxic protease inhibitor from Penicillium sp. These findings support its continued development as a therapeutic candidate against HAT, pending further structural and mechanistic characterization.
Source:
Soerjawinata, W., et al., 2025. Production of Protease Inhibitor With Penicillium sp. — Optimization of the Medium for Growth in Pellet Form and Cytotoxicity Testing
Read the paper here!
Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)
