Data Spotlight
Insights into C. ljungdahlii Serum Bottle Cultivation Using Scattered Light Monitoring
Background
The serum bottle is a widely used vessel type that is sealed from external gas exchange. It can be flushed with defined gas mixtures in a controlled way, which makes it especially useful for gas fermentation processes and anaerobic cultivations. These processes, often carried out with autotrophic microorganisms such as the well-studied acetogen Clostridium ljungdahlii, attract a growing interest because they combine two major advantages: Reducing greenhouse gas emissions through carbon fixation from industrial off-gas and producing valuable products like fuels and chemicals.
Despite their advantages, serum bottles have some major disadvantages, as well. They operate as closed systems, which means the gas supply is finite, and the way gases move between the gas and liquid phases plays a big role in how well microorganisms grow. At the same time, serum bottle experiments are still largely considered “black boxes.” There is little to no online monitoring of what happens inside, and disruptive offline sampling is often avoided due to the risk of oxygen contamination.
Introducing monitoring tools for serum bottle cultivations can therefore provide important insights into gas transfer and microbial performance, and ultimately help advance the development of sustainable gas fermentation technologies.
In this study, scatterred light measurements of the Cell Growth Quantifier (CGQ) were applied in combination with online pressure measurement to monitor gas fermentations of Clostridium ljungdahlii cultures in serum bottles. Serum bottle adapters were used to attach the sensor to the side of the vessel to ensure optimal read outs.
Results
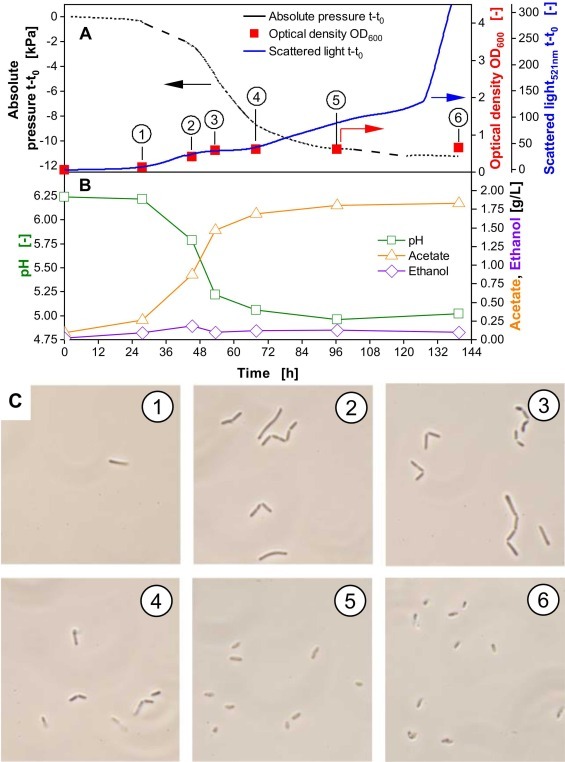
Cultivation of Clostridium ljungdahlii in re-gassed closed serum bottles. Biomass was measured online with the CGQ (scattered light, 521nm, blue) and offline by manual sampling and OD measurements with a photometer (optical density, OD₆₀₀, red). Absolute pressure changes inside the serum bottle were measured online, as well (black).
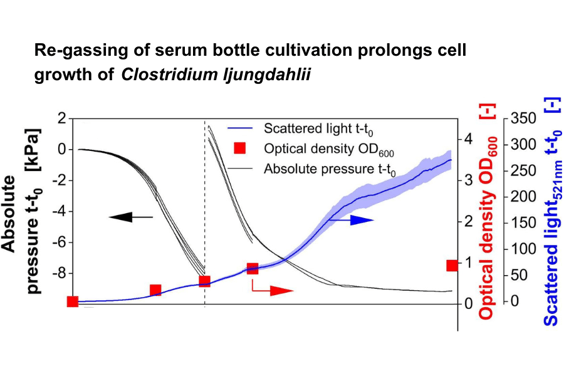
In this experiment, online and continuous monitoring techniques demonstrated the limitations that occur when the c-source is offered in a batch gaseous phase and why continuous re-gassing can improve cultivation conditions. During the first 48 h, the scattered light signal from the CGQ closely matched the offline measured OD₆₀₀. After renewing the gas phase at 48 h, an accelerated decrease in absolute pressure was observed, highlighting that the culture had previously been limited by CO transfer due to reduced headspace pressure. Restoring the CO partial pressure improved gas transfer and growth, which was reflected in the faster pressure drop. After 60 h, this decrease began to slow, and by 66 h, the online biomass signal deviated from the offline OD₆₀₀, likely due to carbon limitation and onset of starvation. To better understand this deviation, additional cultivation experiments with microscopic analyses at different time points were performed.
In this experiment, microscopic images of cells in the cultivation broth were captured to investigate the impact of cell size on scattered light and offline optical density (OD600). During the first 52 hours of cultivation, offline OD600 measurements increased steadily and correlated well with the online scattered light signal. Microscopic images 1–3 (C; sampling time points indicated by the black-encircled numbers) show a rising number of rod-shaped cells.
During the subsequent cultivation phase, growth stagnated, as indicated by stable OD600 values. However, the scattered light signal continued to increase, though moderately between 68–127 hours. Microscopic images 4 and 5, taken during this phase, reveal a reduction in cell size, likely due to starvation. Because backscattered light is strongly influenced by particle size, this continued increase reflects these morphological changes. In contrast, OD600, as an absorbance-based measurement governed by Lambert-Beer’s law, primarily reflects particle concentration. Since growth has plateaued, the OD600 signal remains constant.
After 127 hours, a sharp rise in the scattered light signal is observed, while the offline OD600 remains unchanged (see A). This increase in scattered light may result from morphological changes associated with spore formation, as C. ljungdahlii is known to produce endospores. These observations highlight the potential of the presented online monitoring technology to detect subtle morphological changes that are not captured by conventional OD600 measurements.
Materials & Methods
Experiments were conducted with Clostridium ljungdahlii in 250 mL serum bottles containing 20 mL of ATCC medium. The cultures were adjusted to an initial pH of 6.0 and inoculated to an optical density (OD₆₀₀) of 0.04–0.06. Prior to inoculation, the serum bottles were sealed with butyl rubber stoppers and flushed with the appropriate gas mixture to establish anaerobic conditions. The headspace of each bottle was then filled with 25 % carbon monoxide and 75 % nitrogen. Incubations were carried out at 37 °C with agitation at 100 rpm on an orbital shaker (diameter 50 mm).
For online monitoring of cell growth, the Cell Growth Quantifier equipped with serum bottle adapters was used. Backscattered light was measured through the side of the serum bottle at a wavelength of 521 nm, with a measurement interval of 5 s. Measurements were performed in biological duplicates.
Conclusion
Scattered light measurements provided continuous online data on biomass formation and revealed morphological changes that were not detectable through offline OD₆₀₀ measurements. This highlights the broader potential of online monitoring beyond simple biomass tracking. In this study, the approach was used to better understand and improve the cultivation of C. ljungdahlii in closed serum bottles. Since morphological changes often coincided with substrate limitations, they served as an indicator of suboptimal culture conditions. Microscopy confirmed a correlation between these morphological shifts and unexpected increases in backscatter signals that deviated from offline OD readings. By repeatedly re-gassing the cultures, substrate availability was restored and unfavorable conditions prevented. Thus, the non-invasive backscatter signal from the CGQ proved valuable not only for monitoring cultivation status but also for providing actionable insights to maintain stable growth. What might initially appear as a limitation of backscatter monitoring therefore reveals a novel advantage of the method.
In combination with absolute pressure monitoring, scattered light measurements provide detailed insights into gas fermentation in serum bottles. Substrate limitations become apparent through unexpected increases in backscatter signals and can be mitigated by adapting the gassing strategy. This enables more reliable screening tasks and microbial strain characterization, while reducing the risk of misinterpretation and increasing efficiency.
Source
Schick et al., 2025. Effects of carbon monoxide supply on gas fermentations in serum bottles investigated by online monitoring of gas transfer rates. Biochemical Engineering Journal 222. https://doi.org/10.1016/j.bej.2025.109838
Have questions about your application?
Let’s work together to find a solution that works best for you.
From Estimation To High-Resolution Growth Curves


Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)