
Bioreactors - The Gold Standard
Bioreactors are commonly used for protein production due to their precise monitoring and control capabilities. In contrast, shake flask experiments often lack these features, making them less suitable for generating scalable and reliable results. As a result, bioreactors are typically preferred even in early stages of process development, as they offer better reproducibility and are more effective in optimizing product titers.
Scaling Up From Shake Flasks
Scaling up from shake flasks to bioreactors presents a critical step in bioprocess development. While shake flasks are useful for early-stage screening, they lack the environmental control necessary for consistent, scalable results. Parameters such as oxygen transfer, pH, and mixing are difficult to manage in shake flasks, often leading to suboptimal conditions. In contrast, bioreactors allow precise control over these variables and commonly operate in fed-batch mode rather than simple batch mode. This approach supports more robust biomass formation, especially when sugar feeding is adapted to match the cells’ exponential growth rate. Such tailored feeding strategies enhance overall productivity. Additionally, when working with Pichia pastoris and methanol induction for protein production, bioreactors offer the advantage of controlled methanol addition. Repeated small doses help maintain a stable methanol concentration—avoiding the toxicity of high spikes while still sustaining induction levels—ultimately improving both cell viability and product titers.
Therefore, bioprocess optimization is often still performed in bioreactors rather than shake flasks, as the conditions between these systems differ significantly, making direct data transfer unreliable. However, establishing a reliable scale-down model in shake flasks is highly desirable. A reliable shake flask system that accurately reflects key bioreactor parameters can serve as a valuable medium-throughput platform, enabling faster, cheaper and more effective process optimization.

A typical bioreactor process is tightly controlled, with continuous monitoring of key parameters such as dissolved oxygen (DO), pH, temperature, and agitation speed. Nutrient levels—particularly carbon and nitrogen sources—are also tracked to assess metabolic activity and avoid depletion.
In the process shown here, Pichia pastoris was cultivated to achieve high yields of an active target protein. Key parameters monitored included DO, biomass (via the CGQ BioR), and pH. Antifoam was added to prevent excessive foam formation.
The process began with a batch phase, where cells adapted to the medium and glycerol. After approximately seven hours, a noticeable drop in DO indicated active glycerol metabolism. This was followed by a fed-batch phase, during which glycerol was continuously supplied to boost biomass formation. pH regulation was initiated to maintain a stable environment.
Toward the end of this phase, glycerol feeding was halted to induce a short starvation phase, preparing the cells for the final methanol induction phase. The induction began with a 1% methanol pulse, allowing cells to adapt to the new carbon source. Once the DO dropped—signaling active methanol metabolism—and recovered—indicating substrate depletion—a continuous methanol feed was started to sustain high-level protein expression.
For Scaling Up: Shake Flask Processes Need to Mimick Bioreactor Processes
.png?width=1312&height=481&name=graph-BRAIN-story%20(1).png)
A glycerol fed-batch phase was introduced to the shake flask cultivations before the methanol production phase started.
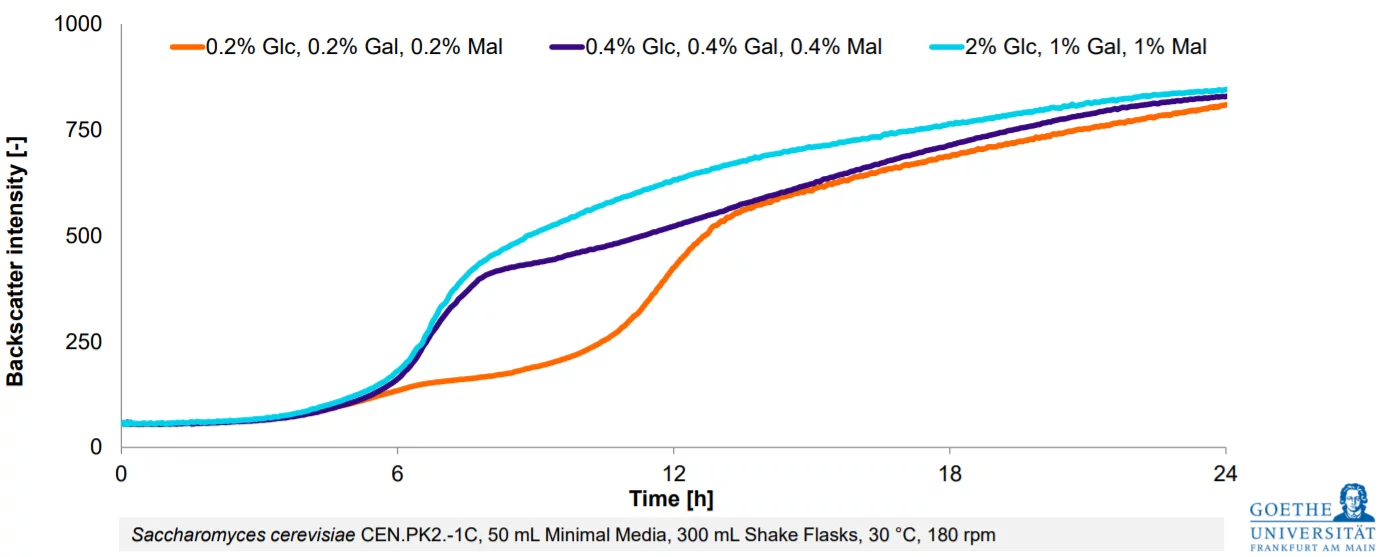
In this study, a shake flask process was designed to closely mimic a typical bioreactor workflow. Following an initial batch phase with 1% glycerol, biomass and dissolved oxygen (DO) were monitored—similar to the bioreactor setup, though without active pH control or antifoam addition.
Next, a glycerol fed-batch phase was implemented. While continuous substrate feeding is uncommon in shake flasks due to the closed system, the Liquid Injection System enabled a controlled and consistent glycerol supply. The feeding solution contained 10% glycerol in 0.1 M K phosphate buffer pH 6.0. The buffered solution significantly stabilized the pH of the medium. Despite the lack of pH monitoring, this setup successfully mirrored the bioreactor conditions, leading to a noticeable increase in biomass.
The final methanol induction phase began with a 1% methanol shot, followed by an adaptation period. As in the bioreactor, DO levels were used to trigger further methanol additions: each time DO recovered—signaling that the cells had metabolized the available methanol—a new dose was introduced. This approach helped maintain a stable methanol concentration, supporting consistent protein expression.
By combining DO monitoring with smart substrate feeding—enabled through the LIS and triggered directly by the DO signal—the shake flask process closely reflected key aspects of the bioreactor setup. This allowed for successful replication of the most essential process elements: Continuous glycerol feeding and precisely timed methanol additions, both vital for high-yield protein production. Implementing this approach in shake flasks enabled an effective scale-down of the bioreactor process, making it easier to conduct tests and optimize conditions at a smaller scale—saving time, reducing costs, and accelerating development.
Volumetric yield increased from 3.8 µg/ml to 56.5 µg/ml in shake flasks

In this study, several strategies were implemented to enhance protein yield in shake flask experiments. Traditionally, Pichia pastoris cultures grown in batch mode with a single methanol addition for induction yield limited amounts of active protein. To overcome this, a series of optimizations were introduced, resulting in a substantial increase in product titer. A major improvement was achieved through DO-based methanol feeding, where methanol addition was adjusted in response to the culture’s oxygen consumption—an indirect indicator of metabolic activity. This dynamic feeding approach helped maintain an optimal methanol concentration, supporting sustained protein expression while avoiding the inhibitory effects of methanol accumulation. Furthermore a constant glycerol fed-batch feed was implemented to support the biomass production phase.
Altogether, these process improvements—centered around adaptive and growth-aligned feeding strategies—resulted in a 15-fold increase in product titer, reaching 56.5 µg/mL from an initial 3.8 µg/mL. This demonstrates that even within the limitations of shake flask systems, significant gains in productivity can be achieved. Moreover, implementing such strategies at the shake flask scale helps bridge the gap to bioreactor-scale processes, making scale-up and scale-down more predictable and reducing the risk of unforeseen challenges.
This study demonstrates that thoughtful optimization of shake flask processes can lead to significant improvements in protein production, even without the use of bioreactors. By implementing controlled methanol induction and growth-adapted feeding strategies, product titers were increased substantially. These results highlight the importance of aligning nutrient supply with cellular metabolism to maximize yield. Importantly, fully exploiting the potential for optimization at the shake flask level lays a strong foundation for successful scale-up, as it helps identify robust, reproducible conditions that translate more effectively to bioreactor systems. Maximizing process performance early on not only boosts productivity but also reduces risks and surprises during upscaling.
From Estimation To High-Resolution Growth Curves
Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)

Want Results Like These?
We will work with you on a solution that works best for your application.
